Publication
Article
Family Practice Recertification
A Young African-American Male with Dyspnea on Exertion and Cough
Author(s):
The statement that there is no rule without exception is arguably more true in cases of sarcoidosis than in almost any other condition.

James D. Collins, MD
This 30-year-old, muscular black male was in excellent health until 2 months ago, when he developed gradually increasing dyspnea on exertion with a reduced exercise tolerance — from being able to run a few miles, to only being able to climb a few steps. He developed a productive cough of some sputum that was greenish in color.
With the onset of his symptoms, he stopped smoking his usual 1 pack of cigarettes per day. Over the previous month, he developed a non-tender and non-puritic skin rash over his neck and experienced weight loss between 5-10 lbs, despite having a good appetite.
Physical examination revealed a blood pressure of 150/70 mm Hg, temperature of 98.4°F, pulse of 82 bpm, respiratory rate of 19.0 breaths per minute, and weight of 180 lbs. The patient’s lung exam was notable for wet rales, and a pulse oximetry revealed his oxygen saturation to be 92% on room air.
Radiographic Findings
The patient’s posterior-anterior (PA) chest radiograph displayed the elevated right shoulder as compared to the lower left shoulder; diffuse alveolar infiltrates throughout the lung fields, most markedly in the right middle lobe, but with no portion of the lungs spared; asymmetric trapezius muscles, where the right was greater than the left; and lobulated, dense, enlarged lymph nodes obscuring detail of the cardiomediastinal structures (Figure 1). The osseous structures were within normal limits.1-3
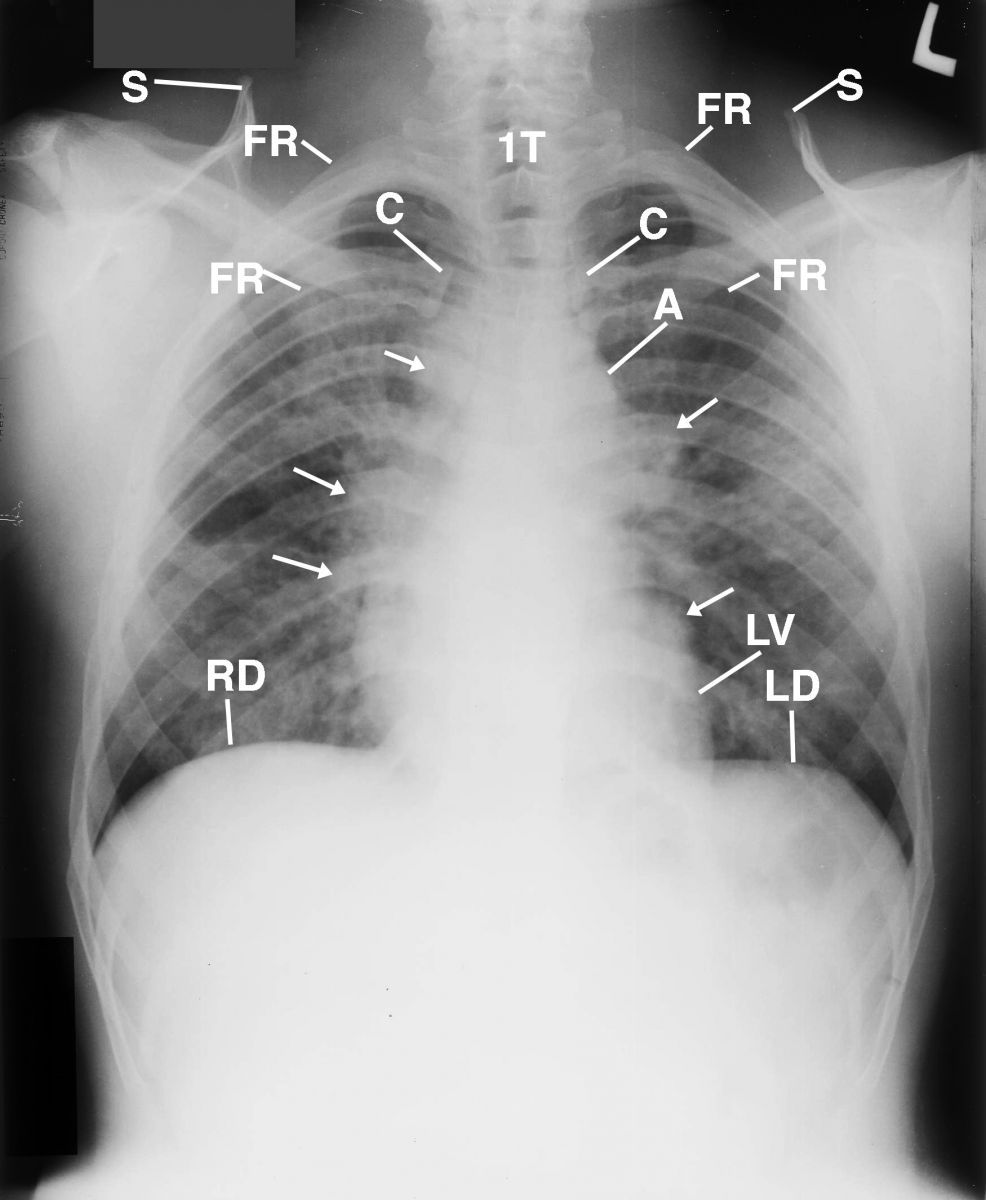
Figure 1 This is a posterior-anterior (PA) chest radiograph that displays the elevated right shoulder as compared to the lower left shoulder; diffuse alveolar infiltrates throughout the lung fields, most markedly in the right middle lobe but with no portion of the lungs spared; asymmetric trapezius muscles, where the right was greater than the left (not displayed); and lobulated dense lymph nodes (arrows) obscuring detail of the cardiomediastinal structures. A= aorta; C= clavicle; FR= first rib; L= left; LV= left ventricle; RD= right hemidiaphram; LD= left hemidiaphram; S= scapula; 1T= 1st thoracic vertebra.
An inverted image of the PA chest radiograph displayed in Figure 1 was obtained to provide a different perspective to enhance the adenopathy within the hilar region (Figure 2).
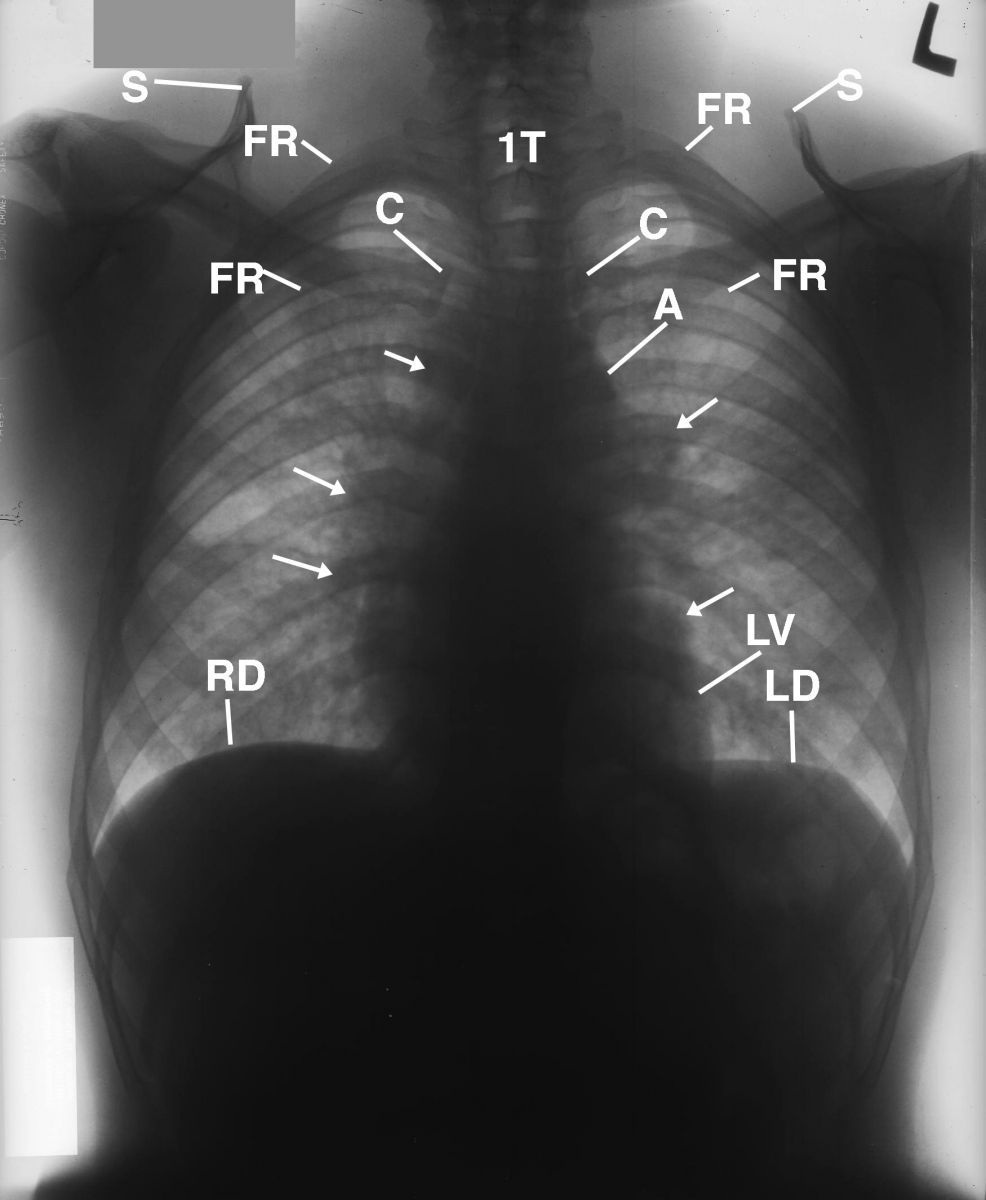
This is the inverted image of the chest radiograph displayed in Figure 1. Observe the lucencies within the lungs reflecting functional lung parenchyma (not labeled).
Figure 2
An enlarged image of the neck was obtained to confirm the absence of cervical lymph nodes (Figure 3). However, the image displayed supernummery/cervical ribs at the C7 transverse processes overlaying the transverse processes of the first thoracic vertebra.
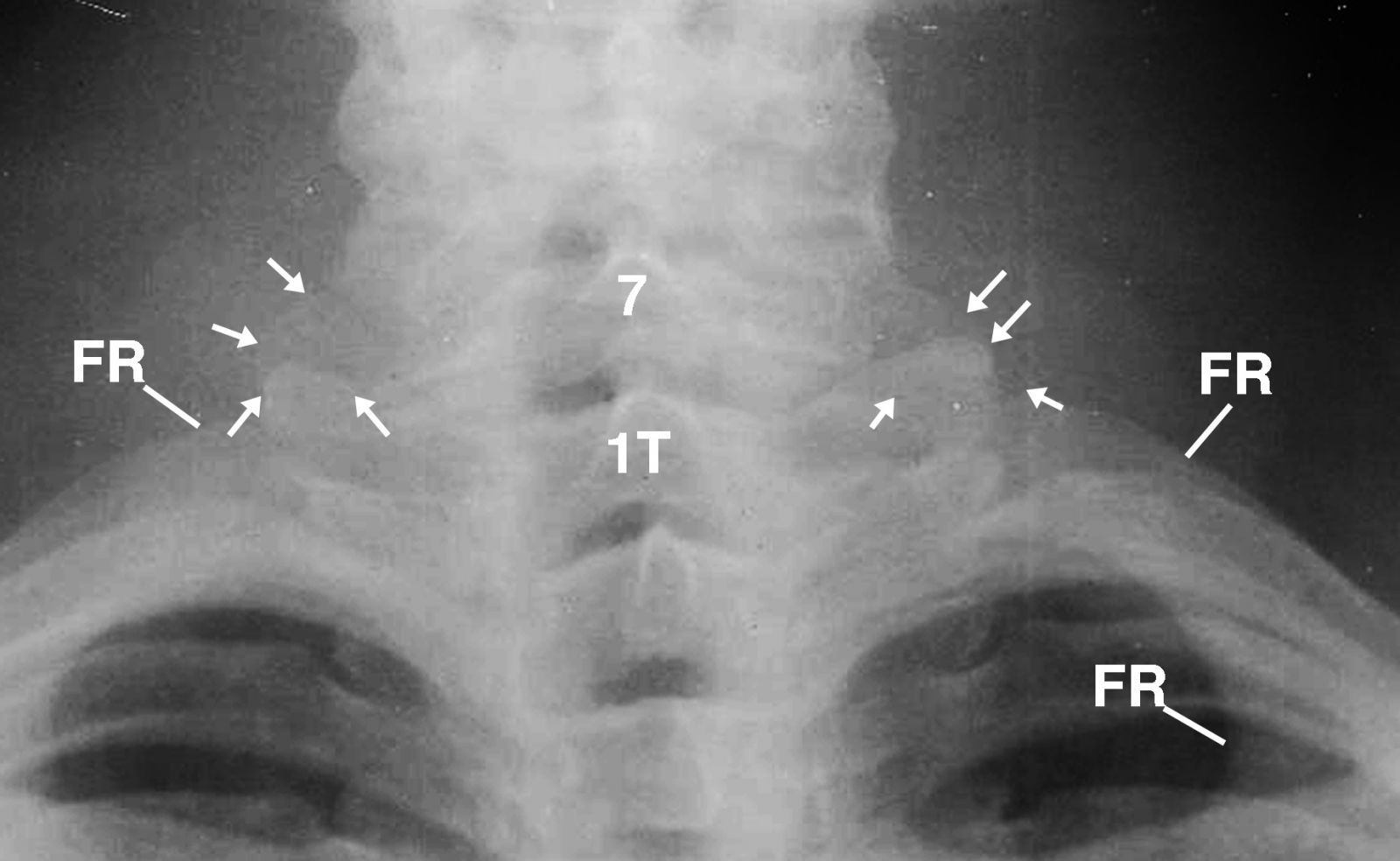
This is an image of the neck that was obtained to confirm the absence of cervical lymph nodes. The image displays supernummery/cervical ribs (4 arrows on each side) at the C7 transverse processes overlaying the transverse processes of the first thoracic vertebra (1T). 7= 7th cervical vertebra
Figure 3
A lateral chest radiograph that crossreferenced the PA chest radiograph was obtained to display the obscured landmark anatomy of the lungs, shoulders, and cardiomediastinal structures secondary to generous muscles, hilar adenopathy and alveolar infiltrations (Figure 4). Air bronchograms were displayed in the region of the right middle lobe bronchus, reflecting alveolar infiltration enhanced by hilar adenopathy.
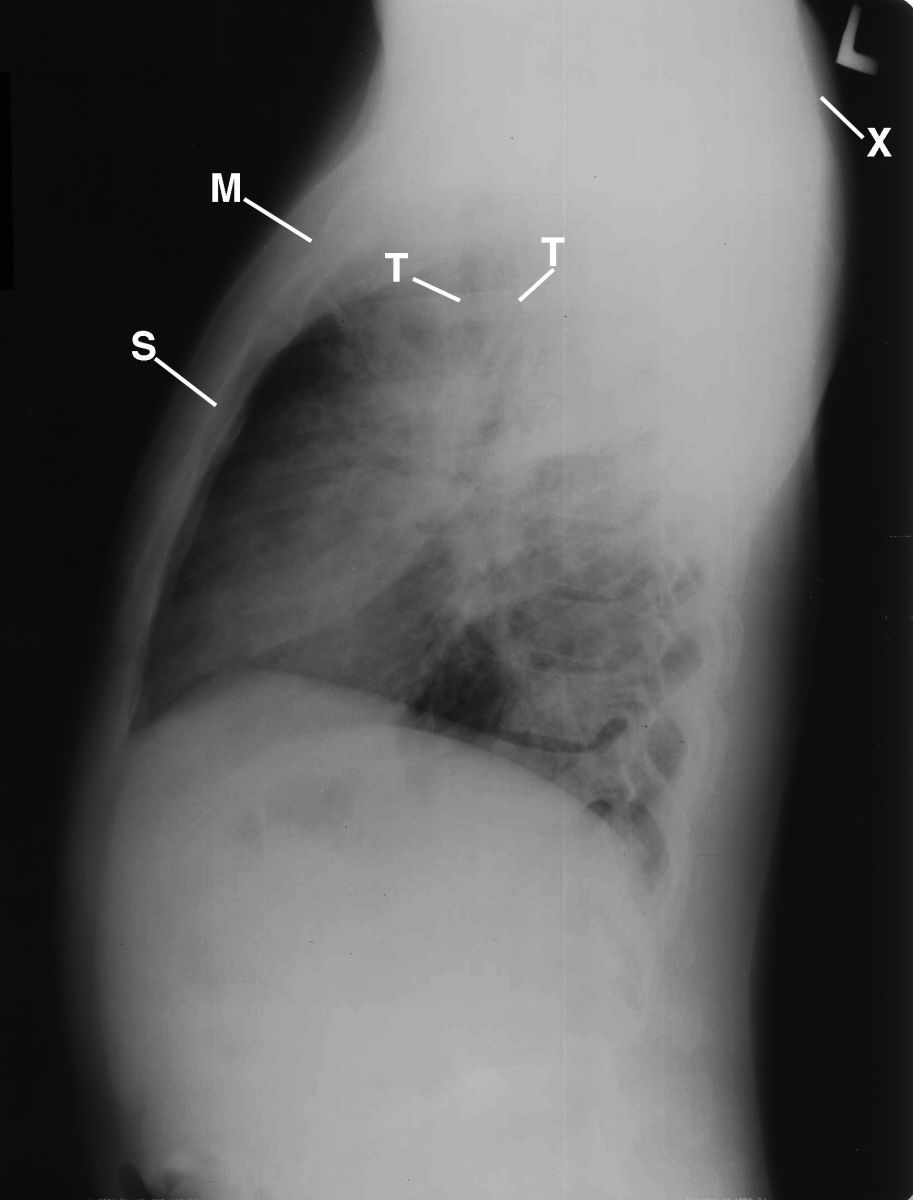
This is a lateral chest radiograph that crossreferences the PA chest radiograph to display the obscured landmark anatomy of the lungs, shoulders, and cardiomediastinal structures secondary to generous muscles, hilar adenopathy, and alveolar infiltrations. Air bronchograms (not displayed) are displayed in the region of the right middle lobe bronchus, reflecting alveolar infiltration enhanced by hilar adenopathy. M= manubrium; S= sternum; T= trachea; X= rounding of the shoulders.
Figure 4
Diagnosis
A percutaneous biopsy of a mediastinal node revealed non-caseating granulomas, which led to a diagnosis of sarcoidosis.
Discussion
Sarcoidosis is an epithelial cell granulomatous disease specific for the reticuloepithelial system, though it may involve any organ. Sites of occurrence include the lymph nodes, lungs, bronchial mucosa, liver, spleen, kidneys, eyes, skin, pleura, lacrimal glands, gastrointentestinal tract, heart muscle, bones and joints, central nervous system, endocrine glands, and hematopoetic system. The common denominator for the occurrence is directly related to the lymphatic system.4
Major considerations in the differential diagnosis of alveolar infiltrates with adenopathy include lymphoma, sarcoidosis, alveolar cell carcinoma, and fungal disease.5 Alveolar proteinosis, which could present with that sort of infiltrate, is not associated with an adenopathy. Lymphoma may cause the alveolar infiltrates by direct infiltration or by predisposing to infection with agents such as cytomegalovirus and pneumocystis, as well as fungal agents such as monilia, coccidiomycosis, histoplasmosis, cryptococcus, and aspergillosis.
The interstitial nodular fibrotic pattern is a more common radiographic picture for sarcoidosis, but the alveolar pattern is not a usual mode of presentation. Thus, parenchymal adenopathy could not be explained by this diagnosis. Fungal diseases by themselves can produce this picture of adenopathy with alveolar infiltrates. Tuberculosis is unlikely in that it is unusual for it to cause significant pulmonary adenopathy in an adult, and an infiltrative pattern such as this would be unusual in the absence of other evidence for tuberculosis. Other possibilities include alveolar microlithiasis, bronchial carcinoma, desquamative interstitial pneumonitis, lymphocytic interstitial pneumonitis, hair spray pneumonia, lipoid pneumonia, psittacosis, asbestosis, and Goodpasture syndrome.
The etiology of sarcoidosis remains a mystery. Some investigators believe there is connection to tuberculosis, but the connection has not been proven.
Three stages of pulmonary sarcoidosis occur: Bilateral hilar lymph node enlargement, with or without involvement of paratracheal nodes; invasion of the lungs with miliary, nodular, reticular, or irregular patchy involvement of the lung parenchyma, as in our patient; and conversion of the pulmonary granulomata into the irreversible fibrosis with hilar scar formation.5
Take-Home Message
The various presentations of the radiographic findings in pulmonary sarcoidosis make the diagnosis difficult. Any pulmonary disease can mimic sarcoidosis, including bronchial carcinoma, tuberculosis, and fibrosis masquerading as asbestosis. Therefore, the radiographic diagnosis of sarcoidosis has to be supported by all clinical methods with follow-up radiographs and biopsy. The statement that there is no rule without exception is arguably more true in cases of sarcoidosis than in almost any other condition.5
References
1. Collins JD. A woman with wheezing, weakness, and weight loss. Family Practice Recertification. 2004 Oct;26(10):24-6.
2. Paul LW, Juhl JH. The Essentials of Roentgen Interpretation (3rd ed.). Hagerstown, MD: Harper and Row, 1972.
3. Weinberger SE. Sarcoidosis. Goldman L, Ausiello D. Cecil Textbook of Medicine. (23rd ed.). Philadelphia: Saunders Elsevier, 2007.
4. Collins, JD, Shaver ML, Disher AC, Batra P, Brown K, Miller TQ. Imaging the thoracic lymphatics: Experimental studies of swine. Clinical Anatomy. 1991;4(6):433-46.
5. Roentgen Diagnosis in Five Volumes. (2nd Amer. ed.). Ed. by Schinz HR, et al. Vol. IV: Pleura, Medistinum and Lungs. New York: Grune and Stratton, 1975.
About the Author
James D. Collins, MD, is Professor and General Radiologist in the Department of Radiology at the UCLA David Geffen School Medicine. He formerly served as Director of the required medical student training for the department and President of the James T. Case Radiologic Foundation. Collins has an extensive background of publications, consultations, and editorial positions, including his current post as the Radiology Editor for the Journal of the National Medical Association. He specializes in bilateral 3-dimensional MRI and MRA imaging of the brachial plexus and has been performing those studies since 1985.





