Press Release
Article
New Phase 3 Data Announced on Efficacy of Tapinarof for Adults, Children with Atopic Dermatitis
Author(s):
These new, positive data from the ADORING Phase 3 development program showed no new safety signals with long-term use as well as a significant reduction in disease burden.
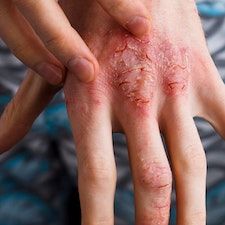
New findings from the phase 3 ADORING trial program suggest that tapinarof (Vtama) cream, 1%, showed positive efficacy and safety data for adults and children 2 years of age or older with atopic dermatitis (AD), according to an announcement by Dermavant Sciences.1
The January 11 announcement by Dermavant contained several positive findings, the principal among them being that 80.7% of participants in the ADORING 3 trial showed a minimum of 75% improvement in their Eczema Area and Severity Index (EASI75) score on tapinarof cream.
The therapy was shown to be well-tolerated over all of the areas treated for AD patients, including sensitive skin and intertriginous regions. The research team also observed no new safety signals with the treatment’s long-term use, with a drug discontinuation rate in ADORING 3 from adverse events (AEs) being 2.6%.
The team also noted no treatment-related serious adverse events, and the majority of AEs being mild to moderate in quality. In ADORING 3, the most common AEs were nasopharyngitis, folliculitis, and upper respiratory tract infection.
The cream itself is a novel, aryl hydrocarbon receptor agonist which is being investigated by the company as a nonsteroidal, once-per-day, topical therapy option for acute treatment and for long-term management of eczema. The drug is approved for plaque psoriasis treatment among US adults and the phase 3 ADORING program features the same strength and the same formulation as that for psoriasis.
“Atopic dermatitis is a burdensome disease, especially for pediatric patients, the most frequently affected patient population,” Eric Simpson, MD, MCR, director of CLEAR Eczema Center at the Oregon Health & Science University, said in a statement. “The integrated analysis data from the ADORING development program are particularly encouraging as they show a high level of efficacy in a diverse patient population, including patients down to 2 years of age, who are in need of a treatment option such as (tapinarof) cream...”
Simpson also noted that if the therapy were approved by the US Food and Drug Administration (FDA) for AD, its effectiveness, safety, and notable rapid onset of pruritus reduction, could represent a new option for both AD patients and their caregivers.
Both the ADORING 1 and the ADORING 2 trials were identical phase 3 studies which the investigators conducted using a randomized, double-blinded, and vehicle-controlled study design. The team looked at the safety data and efficacy data on tapinarof cream, 1%, in participants who were as young as 2 years of age.
Those considered eligible for those 2 trials by the investigators showed a baseline Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score which spanned from 3 (moderate) to 4 (severe). The ADORING 3 trial represented an ongoing, open-label, long-term extension study of both trials which looked at a treatment period of 48 total weeks.
The research team had included 728 subjects in total, and this group was made up of those who completed the ADORING 1, ADORING 2, or Maximal Usage Pharmacokinetics (MUPK) studies. The ADORING 3 extension also included 76 pediatric patients who had enrolled directly in the study after meeting criteria unique from that of ADORING 1 and ADORING 2.
Overall, the research team had noted in ADORING 1 and 2 that continued efficacy was observed past 8 weeks of treatment. The extension study represents a continuation of that success.
“In the analyses of the ADORING development program, approximately 81% of the patient population in the integrated analysis achieved EASI75 using VTAMA cream, and approximately 51% of patients in ADORING 3, including both adults and children as young as 2 years old, achieved complete disease clearance with a vIGA-AD score of 0,” Philip Brown, MD, JD, the Chief Medical Officer of Dermavant, said in a statement.
These findings in the new analyses are set to be included in the company’s sNDA submission for tapinarof cream in the first quarter of 2024.
References
- Dermavant Announces Positive Data from the ADORING Phase 3 Development Program in Atopic Dermatitis with VTAMA® (tapinarof) Cream, 1% in Adults and Children as Young as 2 Years Old. Dermavant Sciences. January 11, 2024. https://www.dermavant.com/dermavant-announces-positive-data-from-the-adoring-phase-3-development-program-in-atopic-dermatitis-with-vtama-tapinarof-cream-1-in-adults-and-children-as-young-as-2-years-old/. Date accessed: January 11, 2024.




