News
Article
Switching with Adalimumab Biosimilar Maintains Benefit for Plaque Psoriasis
Author(s):
Trial data support multiple switching between biosimilar and reference product over a 48-week course for the treatment of psoriasis.
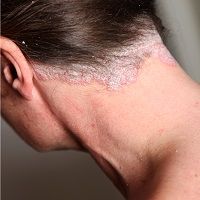
Biosimilar product BI 695501 provided an equivalent pharmacokinetic profile—as well as similar efficacy, immunogenicity and safety—to reference product (RP) adalimumab in patients with chronic plaque psoriasis, according to data.
Research from the phase 3 randomized, double-blind, controlled VOLTAIRE-X trial showed switching from adalimumab RP to BI 695501 was associated with similar clinical outcomes as maintaining the reference product for patients with chronic plaque psoriasis through 48 weeks. The findings support the prior US Food and Drug Administration (FDA) distinction in approval for the biosimilar last year, when the agency defined BI 695501 as the first “interchangeable” monoclonal biosimilar to adalimumab RP.
A multinational team of investigators, led by Alan Menter, MD, of Baylor Scott & White, sought to assess the effect of multiple switching between adalimumab RP and BI 695501 relative to the profile observed in patients who maintained continuous adalimumab RP for the treatment of chronic plaque psoriasis.
“In the US, ‘interchangeability’ is a second regulatory (FDA) approval that allows a biosimilar product to be substituted for the RP without the intervention of the initial prescriber,” investigators wrote. “The FDA criteria for this designation require that a product must have demonstrated biosimilarity to the RP and be expected to produce the same clinical result as the RP in any given patient. Additionally, the risk in terms of safety or diminished efficacy of switching between the biosimilar and the RP should not be greater than the risk of using the RP without switching.”
The VOLTAIRE-X trial included 259 patients treated with adalimumab RP during a run-in period randomized 1:1 to either continuous (n = 118) or switching arms (n = 120). Patients were treated and assessed across 49 sites in the US and Europe from July 2017 – April 2019. Menter and colleagues conducted a run-in period of adalimumab RP 80 mg subcutaneously on day 1, then 40 mg doses every other week from weeks 2 – 12.
Afterward, patients were randomized to either 40 mg adalimumab RP every other week from weeks 14 – 48, or 40 mg BI 695501 weeks 14 and 16, adalimumab 40 mg weeks 18 and 20, then BI 695501 from weeks 22 – 48. Primary trial endpoints included pharmacokinetic outcomes, maximum observed plasma concentration, and area under the plasma concentration – time curve (AUC).
Mean patient age was 44.9 years old; two-thirds (66.0%) were male. Investigators observed a consistent adjusted mean plasma concentration among the switching and continuous arms at 48 weeks (7.08 μg/mL vs 7.00 μg/mL, respectively). Additionally, adjust mean AUC were 2025.8 and 1925.9 μg h/mL, respectively. The confidence interval point estimate for mean AUC ratio was 105.2% and 101.%, falling within the predefined bioequivalence range of 80.0 – 125.0%.
In efficacy outcomes—measured as rates of patients achieving Psoriasis Area Severity Index improvements of 75% (PASI 75)—investigators observed similar benefit across switching (84.8%) and continuous arms (79.0%). What’s more, the proportion of patients with a static Global Physicians Assessment (sGPA) response of ≤1 at week 32 was similar between the 2 arms (70.3% vs 64.7%, respectively).
Regarding safety, 67 (56.8%) patients in the switching arm reported ≥1 treatment-emergent adverse event (TEAE) versus 75 (62.5%) patients in the continuous arm. A majority of TEAEs (58.4%) were not serious in severity. Just injection site erythema (n = 6) and arthralgia (n = 3) were the lone treatment-related TEAEs reported in ≥1% of patients.
Menter and colleagues concluded their findings support the FDA designation of interchangeability with BI 695501 relative to adalimumab RP in the treatment of chronic plaque psoriasis.
“Based on extrapolation of indications, these results provide rheumatologists, dermatologists, and gastroenterologists with data to support the substitution of BI 695501 for adalimumab RP in patients with any of the seven indications for which BI 695501 is approved,” investigators wrote.
Reference
Menter A. Summary of Research: Switching Between Adalimumab Reference Product and BI 695501 in Patients with Chronic Plaque Psoriasis (VOLTAIRE-X): A Randomized Controlled Trial [published online ahead of print, 2023 Oct 24]. Dermatol Ther (Heidelb). 2023;10.1007/s13555-023-00995-z. doi:10.1007/s13555-023-00995-z





