Article
Topline Data from GATHER2 Report Positive Efficacy of Avacincaptad Pegol in GA Treatment
Author(s):
The phase 3 trial met its primary endpoint of mean rate of growth in GA area at 12 months with statistical significance and a favorable safety profile.
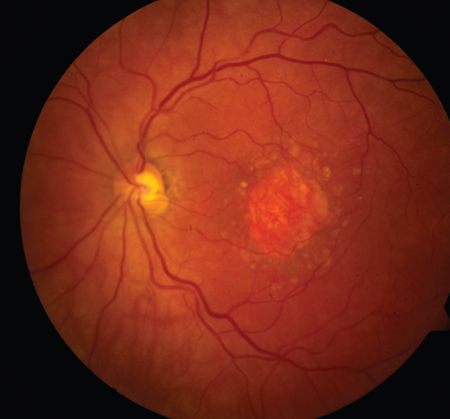
New topline findings suggest avacincaptad pegol (Zimura) reduced the mean rate of growth in geographic atrophy (GA) area over 12 months in the phase 3 GATHER2 trial.
The findings on avacincaptad pegol were considered statistically significant with a favorable safety profile, according to a press release from Iveric Bio.
Within the primary endpoint, data show avacincaptad pegol was linked to a 14.3% reduction (P = .0064) in mean rate of growth in GA area over the 12-month period using square root transformation and a 17.7% reduction (P = .0039) using observed GA area.
“Geographic Atrophy is a devastating and life-altering disease that severely impacts my patients, limiting their ability to drive, read and see the faces of friends and family,” said Arshad M. Khanani, MD, MA, Director of Clinical Research at Sierra Eye Associates in an accompanying statement. “In addition to GATHER1, GATHER2 also meeting the primary efficacy endpoint is great news for patients suffering from geographic atrophy.”
In GATHER2, no events of endophthalmitis, no intraocular inflammation events, and no ischemic optic neuropathy events were reported through month 12. Reported ocular events were most frequently related to the injection procedure.
Moreover, the incidence of choroidal neovascularization (CNV) in the study eye through month 12 was 15 patients (6.7%) in the Zimura 2 mg group and 9 patients (4.1%) in the sham control group.
“Additionally, I am impressed with the safety profile of Zimura in both the GATHER1 and GATHER2 trials, as safety is critically important when evaluating potential treatment options,” Khanani added.
A post-hoc analysis of GATHER2 analyzed the reduction in mean rate of growth in GA area over 12 months for patients receiving avacincaptad pegol by geographic region. Data show the reduction for patients receiving avacincaptad pegol in the US was 25.5% (P-value = .0037) using square root transformation and 32.0% (P-value = .0033) using observed GA area.
Individuals in the US accounted for 42.7% of enrolled patients, but had a mean baseline lesion size that was 13% smaller than patients outside the US. This regional variation may be attributed to disease stage, according to the release.
The company previously hypothesized a greater impact of avacincaptad pegol in earlier stages of GA based on data from GATHER1. Thus, patients in the US in GATHER2 may have additionally been recruited at an earlier stage of the disease due to similar baseline legion size.
The hypothesis will continue to be explored, according to the release.
“We are thrilled to see for the first time an investigational therapy with a statistically significant reduction in the rate of GA progression at the 12-month primary endpoint across two Phase 3 clinical trials,” said Glenn P. Sblendorio, Chief Executive Officer of Iveric Bio in the release. “The results from GATHER1 and GATHER2 and our Special Protocol Assessment with the FDA provide the basis for an NDA, which we are planning to submit by the end of first quarter of 2023.”
The full results of GATHER2 are expected to be presented at the 2022 American Academy of Ophthalmology Annual Meeting.





