Article
Dapagliflozin Helps Prevent HF Hospitalization in T2D without CVD
Author(s):
SGLT-2 inhibitors appear to have a more consistent effect on prevention of HF and renal outcomes than on atherosclerotic CV events, say study authors.
DECLARE-TIMI 58 trial - dapagliflozin vs placebo
Figure. (Please click to enlarge)
Dapagliflozin, a sodium glucose cotransporter-2 (SGLT-2) inhibitor, may help prevent hospitalization for heart failure (HF) and may decrease progression of kidney failure in patients with type 2 diabetes (T2D) at high risk for atherosclerotic cardiovascular disease (ASCVD) but without established disease, according to results from the Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial. The study was published online on November 10, 2018 in the New England Journal of Medicine.1
“In the context of previous cardiovascular outcomes trials with [SGLT-2 inhibitors] empagliflozin and canagliflozin, the DECLARE–TIMI 58 trial supports clear patterns of effect,” wrote first author Stephen D Wiviott, MD, of Brigham and Women’s Hospital, Harvard Medical School (Boston, MA) and colleagues with the DECLARE-TIMI 58 study.
“SGLT2 inhibitors have a more robust and consistent effect on the prevention of heart failure and renal outcomes than on atherosclerotic cardiovascular events,” they explained.
While SGLT2 inhibitors may be associated with moderate protection from MACE in patients with established ASCVD, this effect has yet to be shown in patients at high risk for CVD but without established disease, they added.
Cardiorenal benefits
Dapagliflozin is one of four SGLT2 inhibitors approved by the FDA for treatment of T2D. Studies have suggested that this class of antihyperglycemics has cardio- and renal-protective properties. In 2016, empagliflozin became the first antidiabetes drug to receive an expanded indication for treating ASCVD in patients with T2D and established ASCVD. That decision came largely on the heels of the EMPA-REG trial, which linked empagliflozin to decreased CV death in patients with T2D and ASCVD.2
DECLARE-TIMI 58 was a randomized, double-blind placebo-controlled, phase 3 trial that involved 882 sites in 33 countries. It included 17 160 participants aged 40 and over with T2D, of whom 59.4% had multiple risk factors for ASCVD but had not yet developed it. Participants were randomized to 10 mg dapagliflozin daily or placebo.
Primary efficacy outcomes: Major adverse cardiac events (MACE: CV death, MI, or ischemic stroke) and a composite of CV death or hospitalization for heart failure.
Secondary efficacy outcomes: Death from any cause and a renal composite (decrease in estimated glomerular filtration rate, new end-stage renal disease, or death from renal or CV causes).
During the follow-up period (median of 4.2 years) 1500 participants experienced MACE and 900 died from CV causes or were hospitalized with heart failure.
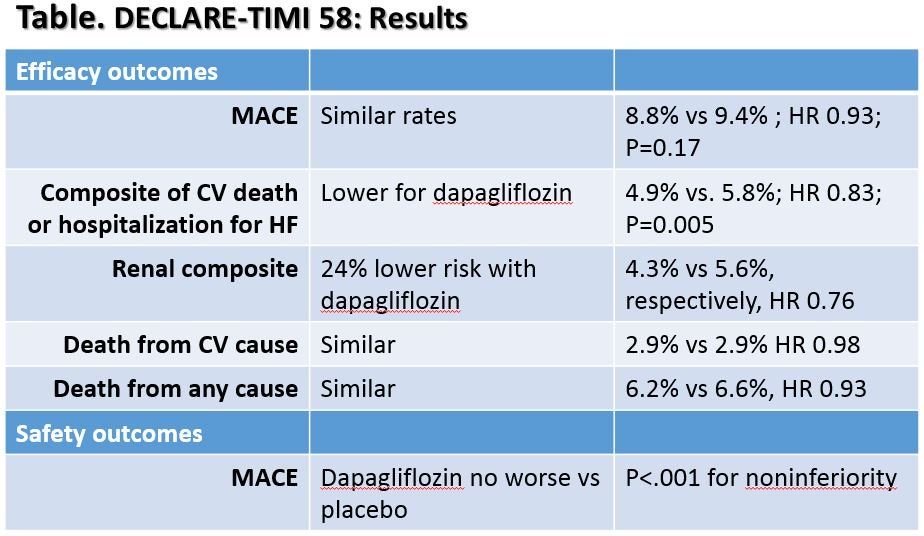
Key results for dapagliflozin vs placebo are presented in the Table seen in the Figure above (please click to enlarge).
Dapagliflozin linked to low risk of HF hospitalization
The authors noted that the lower rate of the composite of CV and hospitalization for HF was largely driven by lower rates of hospitalization for HF with dapagliflozin. Compared to placebo, the dapagliflozin group had 27% lower risk of hospitalization for HF, yet both groups had similar rates of CV death.
They added that results for CV death and death from any cause contrast with results from the EMPA-REG trial, which found lower rates with empagliflozin vs placebo. Differences between the two drugs may explain the effect, they wrote. Also, the two trials had different designs and the DECLARE-TIMI 58 also lower overall mortality rates than the EMPA-REG trial, both of which could affect results. However, the reason for the discrepancy remains unknown, and could also have been due to chance.
Take Home Points:
- DECLARE TIMI 58 found that dapagliflozin may prevent hospitalization for HF and decrease progression of kidney failure in patients with T2D at high risk for ASCVD but without established disease
- MACE, death from CV events, and death from any cause were similar with dapagliflozin vs placebo
- SGLT2 inhibitors may have a stronger effect on the prevention of heart failure and renal outcomes than on MACE in patients with T2D and without established CVD
References:
1. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018. Nov 10.
2. US FDA. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. Dec 2016. Accessed Dec 6 2018 at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm531517.htm





