Article
ACR, Vasculitis Foundation Updates Kawasaki Disease Guidelines
Author(s):
“Kawasaki disease continues to be an area of evolving understanding in clinical treatment,” said Mark Gorelik, MD, lead investigator of the guideline.
This article was originally published on Practical Cardiology.
New guidelines for the management of Kawasaki disease have been released by the American College of Rheumatology (ACR) in partnership with the Vasculitis Foundation (VF).
Released on March 8, the guideline, which addresses diagnostic issues relating to Kawasaki disease, the treatment of high-risk patients, and the management of convalescent patients, is the final companion to three other ACR/VF vasculitis guidelines released in July 2021.
“Kawasaki disease continues to be an area of evolving understanding in clinical treatment,” said Mark Gorelik, MD, an Assistant Professor at Columbia University Vagelos College of Physicians and Surgeons in New York and the lead investigator of the guideline, in a statement. “There are various degrees of severity in this disease and a set of complications and therapies that rheumatologists should be aware of. These guidelines will help clinicians better treat patients by augmenting existing guidelines from the American Heart Association, especially for complex patients seen by rheumatologists.”
Within the guideline, which was composed using 16 clinical questions addressing the diagnostic testing, treatment, and management of Kawasaki disease, the ACR and VF created 1 good practice statement, 11 recommendations, and 1 ungraded position statement to provide clinicians with contemporary guidance for management of Kawasaki disease and clinical scenarios of suspected Kawasaki disease.
The good practice statement addressed the use of intravenous immunoglobulin (IVIG), which it purports should be viewed as the standard-of-care for initial treatment of Kawasaki disease. The ungraded position statement within the guideline outlines the role of either nonglucocorticoid immunosuppressive therapy or glucocorticoids for patients with acute Kawasaki disease and persistent fevers after repeated treatment with IVIG. The 11 recommendations are listed in the infographic below:
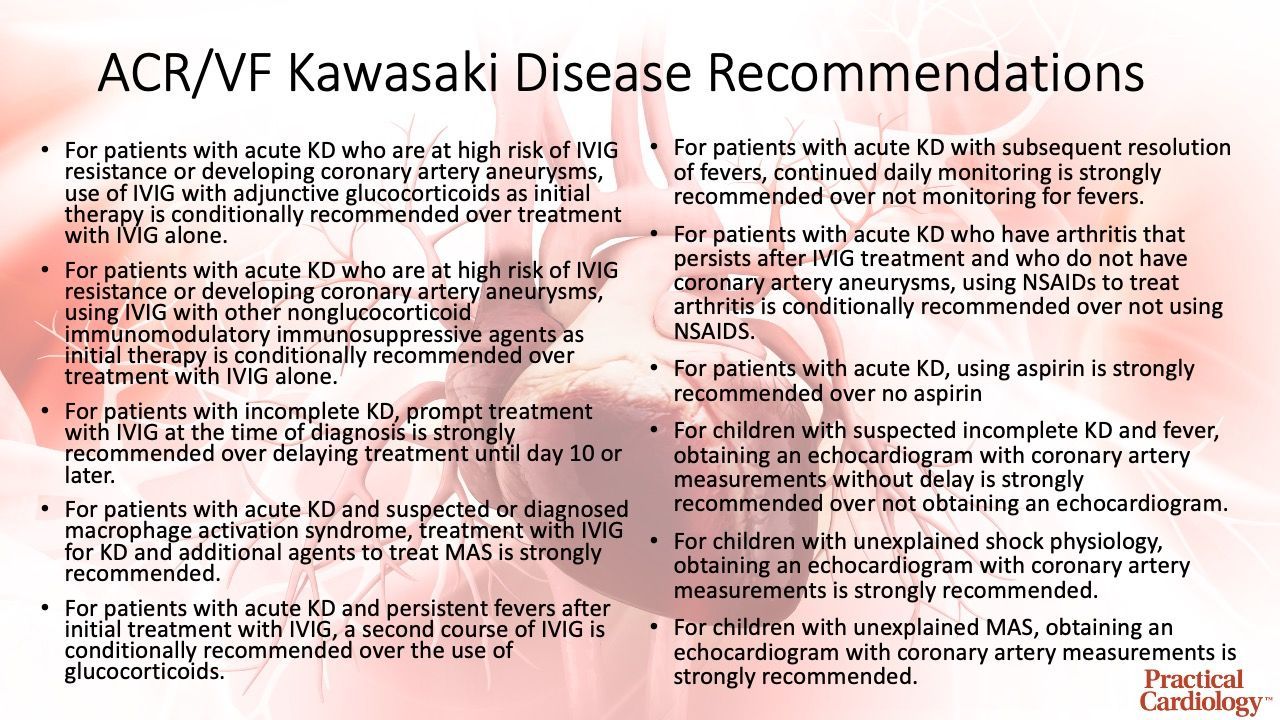
The release from the ACR pointed out the development of this guideline began prior to the COVID-19 pandemic and, during the pandemic, reports of a novel multisystem inflammatory syndrome in children associated with COVID-19 with features similar to Kawasaki syndrome prompted the ACR to release separate clinical guidance on MIS-C.
“Kawasaki disease is the leading cause of acquired heart disease in children,” said Joyce Kullman, Executive Director of the Vasculitis Foundation. “This guideline will hopefully take the guesswork out of determining which treatments might work best for newly diagnosed patients, or patients who have been under treatment for a while without success.”
This guideline, titled “2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Kawasaki Disease,” was published by the ACR and VF.




