News
Article
AGA Clinical Guidelines on the Management of Pouchitis and Inflammatory Pouch Disorders
Author(s):
Recent recommendations from the AGA may help gastroenterologists manage the rising rate of pouchitis with the aid of clinical evidence.
Taha Qazi, MD

Despite the advent of biologics, nearly 20-30% of patients with ulcerative colitis (UC) will require colectomy. Total proctocolectomy with IPAA remains the surgical choice of management for ulcerative colitis and complications related to the disease. The procedure is typically multi-staged, and there are both procedure associated and patient-specific considerations that are important in the ultimate success of the surgery.
Following surgery, most patients experience great functional outcomes, with a gradual improvement in stool frequency and consistency. Most patients average between 4-8 bowel movements daily, and 1-2 nocturnal bowel movements with semi-liquid consistency. Fecal incontinence and seepage can occur in the long term and should be discussed with patients.
Despite most patients having good long-term functional outcomes, complications can occur; the most common complication is the inflammatory complication of pouchitis, which may a significant burden in terms of both quality of life and care costs. Studies suggests that nearly half of patients develop pouchitis within the first 2 years after colectomy, and roughly 80% develop pouchitis at some point during their post-operative course.1,2
Symptoms suggestive of pouchitis or an inflammatory pouch disorder and the need for additional workup include increased bowel movement frequency and urgency, abdominal pain/cramping, blood in stool, and perianal disease. This additional workup generally involves an endoscopic evaluation of the pouch. Additional workup may include imaging evaluations, such as MRI, defocography, or an exam under anesthesia to assess other sources/causes of pouch dysfunction.
In order to provide guidance on the management of inflammatory pouch complications, the authors of the American Gastroenterological Association (AGA) guidelines aimed to provide standardized definitions for the treatment of inflammatory pouch conditions.3 Moreover, by compiling the data from the studies provided, the authors hoped to provide a better treatment paradigm in treating pouch inflammatory disorders.
A crucial aspect of the guidelines are the standardized definitions of pouchitis and inflammatory conditions of the pouch that the authors propose. These pragmatic definitions are not defined on the basis of specific number of episodes or requirement of antibiotics, as this likely represents a continuum.
- Intermittent Pouchitis (IP): Isolated and infrequent episodes of typical symptoms that resolve with therapy (antibiotics)
- Chronic Antibiotic-Dependent Pouchitis (CADP): Recurrent episodes of pouchitis that respond to antibiotics but relapses thereafter, requiring recurrent or continuous antibiotics or advanced therapies
- Chronic Antibiotic Refractory Pouchitis (CARP): Relapsing-remitting or continuous symptoms of pouchitis with inadequate response to typical antibiotics, requiring escalation of therapies
- Crohn’s-like Disease of the Pouch (CLDP):
- Presence of perianal and other fistulizing disease 12 months from final stage
- Stricture of the pouch body or pre-pouch ileum
- Pre-pouch ileitis
Lastly, the authors posed that the treatment goals and targets are clinical remission, and recommendations for endoscopic and/or histologic remission were not considered a critical target.
Table
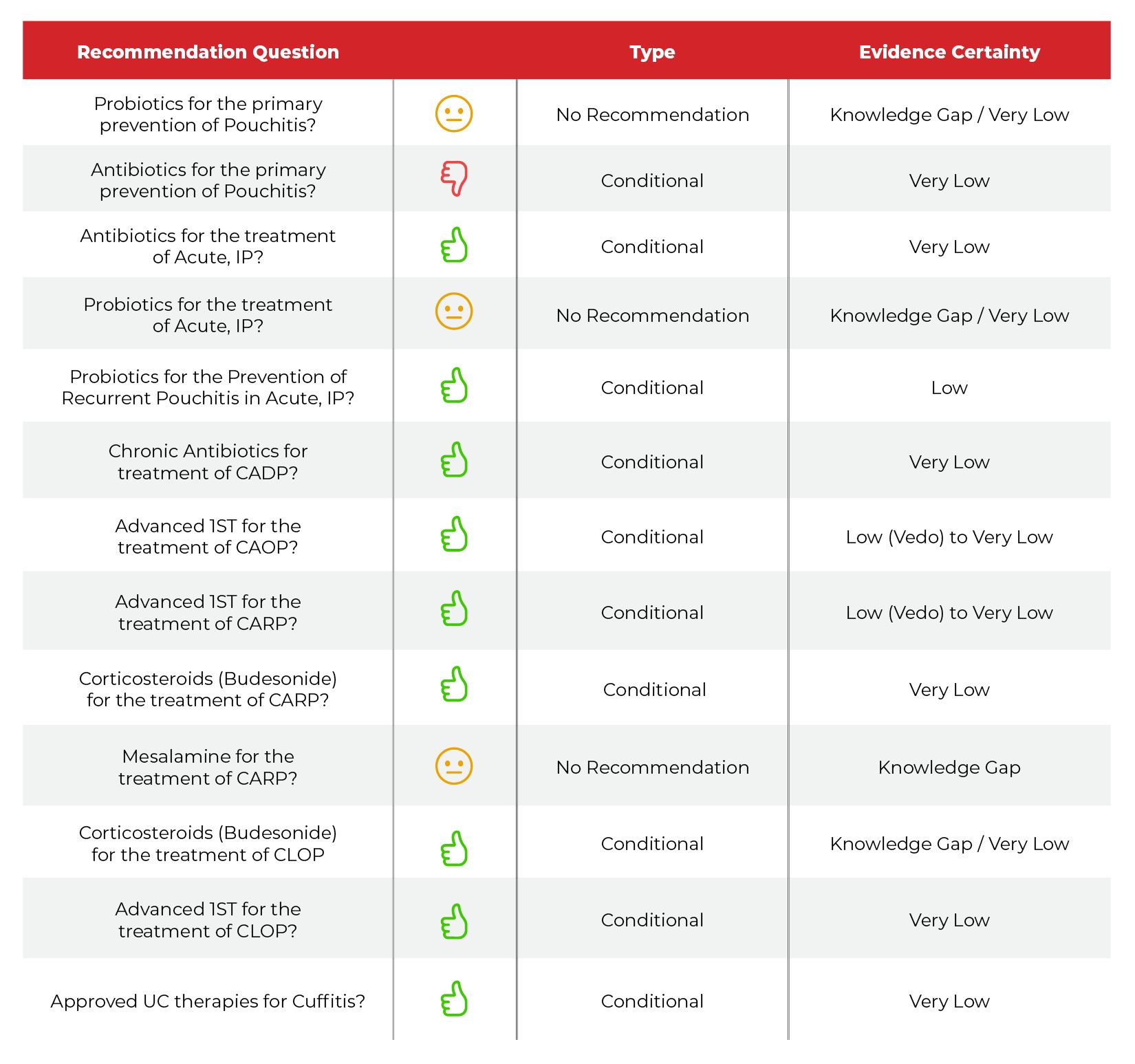
For primary prevention, the authors of the guideline provide no recommendation on the use of probiotics against primary prevention of pouchitis. The authors reported a knowledge gap, with very low-quality evidence. In current practice, most patients undergoing pouch creation do not use of a primary preventative strategy and respond rapidly to an initial course of antibiotics. A daily preventative strategy may not be useful in this setting. Although there is low risk of adverse events, cost of probiotics can be an issue and data on the strains of probiotics that are most helpful has not been studied.
The guideline authors also recommended against the use of antibiotics for primary prevention for similar reasons. Although the authors cited a single study using oral tinidazole with possible benefit, the risk of adverse effects of indefinite antibiotic therapy for the prevention in an asymptomatic patient may be higher compared to sporadic use in setting of acute pouchitis.
The authors do recommend the use of antibiotics for the management of acute, intermittent pouchitis, based on 4 randomized controlled trials as well as 8 observational studies. The pooled response rate in these studies was 65%. The most well-studied agents were ciprofloxacin and/ or metronidazole, singularly or in combination. Additional agents included vancomycin, which was shown to have clinical benefit, as well as rifximin. The typical antibiotic course was 2-4 weeks.
The authors provided no recommendation on the use of probiotics for the treatment of acute intermittent pouchitis, reporting a knowledge gap with low-quality data. Three studies were cited using varying formulations of probiotics with benefit, and although adverse effects are infrequent, cost considerations were significant. Moreover, recommendations regarding benefits of un-studied formulations of probiotics cannot be made. Probiotics may delay effective therapy with antibiotics, which have a greater body of evidence and known clinical efficacy.
However, the authors of the guidelines did suggest that probiotics can be used for the secondary prevention of pouchitis. This is based on the data from 3 randomized, with an 87% lower risk of relapse over 12 months in patients treated with probiotics, specifically the De Simone formulation. The authors of the guidelines did state personal experience temper the use of probiotics, as the reduction reported in the RCTs has not been borne out in real-world practice.
The authors of the guidelines did recommend for the use of chronic antibiotics in the treatment of Chronic Antibiotic Dependent Pouchitis. There is limited evidence and the basis of this recommendation is based on real-world efficacy in the collective experience of the guideline writers. Multiple antibiotic therapies may be effective in this setting, and the authors suggests cycling antibiotics and/or potentially de-escalating to the lowest dose possible with intermittent gaps to minimize adverse events.
The guidelines also suggest for the use of advanced therapies in chronic antibiotic dependent pouchitis (CADP) and chronic antibiotic refractory pouchitis (CARP.) Although much of this is based on the results of cohort studies, it is also based on the recently published EARNEST trial, featuring 102 patients with chronic pouchitis (both CADP and CARP) who were treated with placebo or vedolizumab. Vedolizumab was more effective than placebo in inducing clinical remission (35%) versus placebo (18%). It is important to note that 21% of patients continued to require antibiotics after week 34, and more patients in the vedolizumab treatment arm required antibiotics than in the placebo group, suggesting the ongoing role of anti-microbials in this patient population.
The use of corticosteroids, namely ileal-released budesonide, was suggested by the authors for the management of CARP. This was based on both expert opinion as well as the results of 2 case series, reporting an endoscopic and clinical benefit of ileal-released corticosteroid preparation. The pooled response rates in these case series were 77%. The authors recommended limiting duration to 8-12 weeks with a consideration for steroid-sparing agents.
The authors offered no recommendation regarding the use of Mesalamine for the treatment of CARP. The guidelines did cite 1 study on the use of sulfasalazine in 11 patients with benefit, which may be unique to the use of sulfasalazine due to its known anti-microbial effect.
The authors do recommend the use of ileal-released budesonide for Crohn’s-like disease of the pouch based on no studies, but with indirect data based on expert experience. The goal, once again, is to minimize duration to 8-12 weeks with a consideration for steroid-sparing agents.
Based on 10 cohort studies or case series, the authors also suggested for the use of advanced therapies for Crohn’s-like disease of the pouch, with a pooled response rate of 74%. Pre-colectomy therapies can be recycled. The majority of the studied therapies were anti-tumor necrosis factor (TNF), with smaller number of studies for vedolizumab and ustekinumab.
For the management of cuffitis, the authors suggested therapies that have been approved for the treatment of UC. Topical agents were recommended as first lines, with a suggestion of advanced IST in refractory cases. Data extrapolated from prior AGA guidelines for the management of mild-to-moderate and severe UC, considering that the cuff is a remnant of the rectal mucosa.
A friendly table for the guideline recommendation is provided above. Overall, I want to highlight that the nearly all of these recommendations are conditional and based on low/very low certainty of evidence, from studies of a few patients. This is likely due to the fact that although there is a rising incidence of pouchitis, definitions of disease, outcomes as well as recruitment has been a major issues in clinical trials and studies, including the EARNEST Study.
One of the biggest values of these guidelines is the push to standardize our definitions of inflammatory conditions of the pouch and provide guidance on clear treatment goals, which will aid in future research in this area—a clear unmet need for our patients.
References
- Lightner AL, Mathis KL, Dozois EJ, et al. Results at Up to 30 Years After Ileal Pouch-Anal Anastomosis for Chronic Ulcerative Colitis. Inflamm Bowel Dis 2017;23:781-790.
- Barnes EL, Herfarth HH, Sandler RS, et al. Pouch-Related Symptoms and Quality of Life in Patients with Ileal Pouch-Anal Anastomosis. Inflamm Bowel Dis 2017;23:1218-1224.
- Barnes EL, Agrawal M, Syal G, Ananthakrishnan AN, Cohen BL, Haydek JP, Al Kazzi ES, Eisenstein S, Hashash JG, Sultan SS, Raffals LE, Singh S; AGA Clinical Guidelines Committee. Electronic address: clinicalpractice@gastro.org. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.




