Article
Another Milestone for Acne Vulgaris: New Phase 3 Clinical Trial Begins
Author(s):
Researchers initiated a new phase 3 clinical trial for topical agent olumacostat glasaretil, a potential treatment for acne vulgaris.
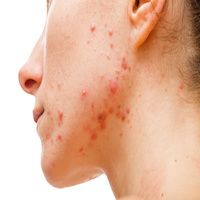
Researchers initiated a new phase 3 clinical trial for topical agent olumacostat glasaretil, a potential treatment for acne vulgaris.
This trial by biopharmaceutical company Dermira aims to evaluate the safety and efficacy of olumacostat glasaretil (DRM01). This novel molecule is specifically designed to reduce sebum (oily secretions) and thus target a fundamental cause of acne — something current topical therapies fail to address.
Despite existing treatment options for acne vulgaris, many people are still seeking safe and effective therapies to relieve their chronic skin conditions.
The clinical program involves two randomized trials, CLAREOS-1 AND CLAREOS-2 that would compare olumacostat glasaretil to placebo and is expected to enroll nearly 1,400 patients at least nine years old who have moderate-to-severe acne vulgaris.
The global program would have at least 100 sites in the United States, Canada, and Australia.
Both trials would randomize at least 700 patients to apply either olumacostat glasaretil at a 5.0% concentration or placebo twice daily for 12 weeks. The US Food and Drug Administration (FDA) has recommended researchers to ensure one of the phase 3 trials to allow patients to apply olumacostat glasaretil or placebo to acne-affected areas on the back, chest, or shoulders. They’d like to see data supporting potential real-world use of the topical agent.
The research team is advised to keep this program’s inclusion criteria consistent with the two earlier phase 2 trials:
· Patients should have a minimum of 20 inflammatory lesions and 20 non-inflammatory lesions
· Investigator’s Global Assessment (IGA) score of three or four on a five-point scale representing clear skin to severe disease
According to the experts, the primary endpoints of both trials will assess the “absolute changes” in inflammatory and non-inflammatory lesion counts on the face as well as the percentage of patients who attained at least a two-grade improvement on the IGA scale at the end of the 12-week period.
The program will also include an open-label study, CLARITUDE, to analyze long-term safety of olumacostat glasaretil. For up to another 36 weeks, patients from either phase 3 studies will be allowed to continue receiving treatment. Officially expect topline results for CLAREOS-1 and CLAREOS-2 in the first half of 2018.
Related Coverage:
FDA Gives Thumbs Up to New OTC Acne Treatment





