News
Article
Apremilast Improves Outcomes in Scalp Psoriasis
Author(s):
Key Takeaways
- Apremilast significantly reduced scalp pruritus and improved quality of life in patients with moderate-to-severe scalp psoriasis.
- The Phase 4 study showed sustained improvements in pruritus and quality of life through Week 52.
The oral therapy may address limitations including difficulty in application and adverse events with topical therapies.
Credit: Adobe Stock
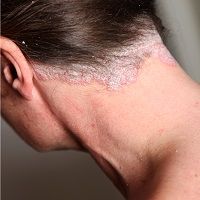
Apremilast (Otezla), an oral phosphodiesterase 4 (PDE4) inhibitor, was efficacious in treating moderate-to-severe scalp psoriasis, with significant improvements in scalp pruritus, quality of life, and clinical severity scores.1
“Scalp psoriasis is a challenging condition that significantly impacts patients' quality of life due to severe pruritus, visible scaling and social stigma. Current treatments, including topical therapies, phototherapy and systemic agents, often have limitations due to side effects, difficulty in application or lack of efficacy, especially in areas like the scalp,” lead investigator Sara E. Cerminara, Department of Dermatology, University Hospital of Basel, Basel, Switzerland, and colleagues wrote.1
Data from a Phase 4, multicenter, randomized, placebo-controlled study were published in a letter to the editor in Journal of the European Academy of Dermatology and Venereology. The study’s primary endpoint was the proportion of patients achieving a ≥20% reduction in scalp pruritus, as measured by the visual analogue scale (VAS), at Week 16. Secondary outcomes included overall reductions in general pruritus, improvements in quality of life (QoL; assessed by the Dermatology Life Quality Index, DLQI), and clinical responses based on the Psoriasis Scalp Severity Index (PSSI), Physician's Global Assessment (PGA), and Psoriasis Area and Severity Index (PASI).
The investigators found that at Week 16, 85.7% of patients receiving apremilast experienced at least a 20% reduction in scalp pruritus, compared to 53.5% in the placebo group (P = .020). In QoL measures, 64.3% of apremilast-treated patients showed a significant improvement in DLQI scores (≥5-point reduction), compared to 39.3% in the placebo group. These improvements in both pruritus and quality of life were maintained or further improved through Week 52, after the placebo group crossed over to apremilast treatment at Week 16. The investigators also observed significant improvements in PSSI and PASI scores in the apremilast group through Week 52.1
The safety profile of apremilast was consistent with previous studies, with gastrointestinal (GI) events and headaches being the most common adverse events (AEs). These AEs were generally mild-to-moderate in severity.
The investigators noted that the study had limitations, including the use of a ≥20% reduction in scalp pruritus as a primary endpoint, which has not yet been fully validated as a standard outcome measure for scalp psoriasis. The GI AEs may also influence blinding in the study. They concluded that further studies with active comparators, such as biologics or other systemic agents, are needed to accurately assess apremilast’s comparative benefit for scalp psoriasis.
“In conclusion, apremilast is an effective treatment for scalp psoriasis in respect to pruritus reduction, DLQI improvement and Scalpdex. This study highlights the great impact and necessity of treatment for special locations, which often have a high DLQI but not necessarily a PASI > 10 as in this study,” Cerminara and colleagues concluded.1
Other recent research on apremilast found that individuals treated with oral apremilast for genital psoriasis may experience consistent benefits of this treatment, according to recent findings, with improvements in sexual health, life quality, and overall psoriasis skin clearance.2
“Men and women treated with apremilast, the first oral systemic treatment studied for genital psoriasis, experienced consistent treatment benefits, including improved sexual health and QoL, and achievement of genital and overall psoriasis skin clearance,” lead investigator Joseph F. Merola, MD, MMSc, from the Brigham and Women’s Hospital at Harvard Medical School, and colleagues wrote.2 “While sample size for female patients was lower, results are notable as women with genital psoriasis often experience higher disease burden than men.”




