Article
Apremilast Reduces Oral Ulcers in Behçet’s Syndrome
Author(s):
Apremilast (Otezla, Celgene) resulted in a greater reduction in the number of oral ulcers associated with Behçet’s syndrome than placebo, say researchers recently writing in the New England Journal of Medicine.
(@Leungchopan,AdobeStock)
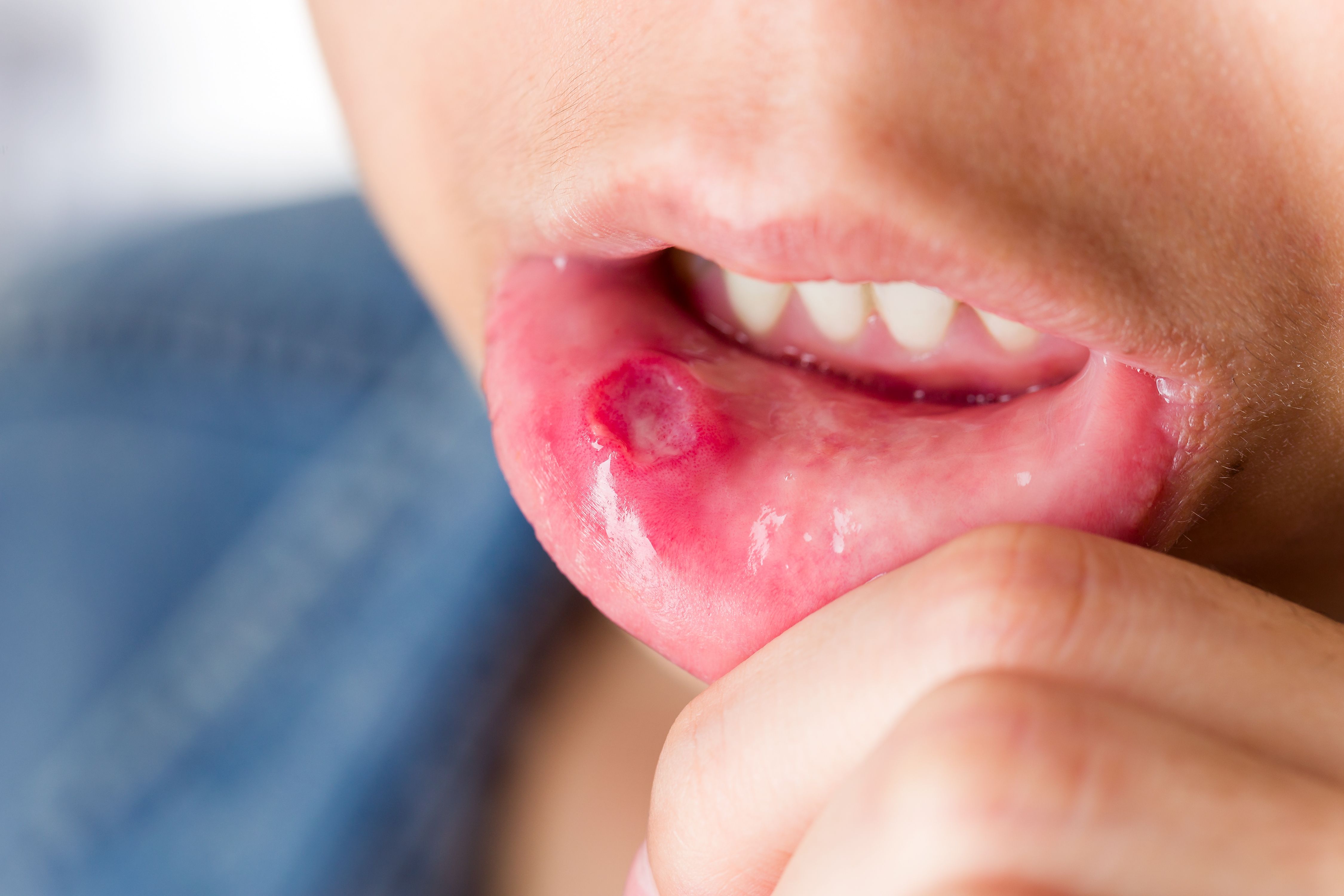
Apremilast (Otezla, Celgene) resulted in a greater reduction in the number of oral ulcers associated with Behçet’s syndrome than placebo, say researchers recently writing in the New England Journal of Medicine.
In Behçet’s syndrome, the first manifestations are often recurrent relapsing and remitting oral ulcers, which cause pain; difficulty in eating, drinking, and talking; and decreased participation in routine daily activities and quality of life. Colchicine is recommended in Behçet’s syndrome as first-line treatment for skin and mucosal involvement, but the efficacy of this drug for the treatment of oral ulcers has not been shown. Apremilast is an oral small-molecule phosphodiesterase 4 inhibitor that inhibits the release of proinflammatory markers such as interleukins 2, 8, 12, and 17 and increases the production of anti-inflammatory mediators. In a phase 2 trial, the drug was shown to decrease the number of oral ulcers, the associated pain, and overall disease activity which can present as seronegative rheumatoid arthritis.
“In the current phase 3 trial, we evaluated the efficacy and safety of apremilast in a larger, geographically more diverse group of patients with Behçet’s syndrome who had active oral ulcers that did not respond to previous treatment with at least one nonbiologic agent such as a topical glucocorticoid or systemic treatment,” wrote the authors of the study, led by Gülen Hatemi, M.D., of the Behçet's Disease Research Center in Turkey.
In the study, 207 patients (mean age 40 years, 61 percent female, mean duration of Behçet's 6.8 years, mean number of active oral ulcers 4.1) with no major organ involvement were randomized 1:1 to receive either apremilast, 30 mg twice daily, or placebo for 12 weeks, followed by a 52-week extension phase.
The area under the curve for the number of oral ulcers during 12 weeks of treatment was 129.5 for apremilast and 222.1 for placebo (least-squares mean difference, −92.6; 95% confidence interval [CI], −130.6 to −54.6; P<0.001). The change from baseline in the Behçet’s Disease Quality of Life score was −4.3 points in the apremilast group and −1.2 points in the placebo group (least-squares mean difference, −3.1 points; 95% CI, −4.9 to −1.3).
“A decrease in the number of oral ulcers and the pain associated with oral ulcers started as early as week one,” the authors wrote. “These results were consistent across subgroups analyzed according to baseline characteristics: disease duration, number of oral ulcers, geographic region, and history of use of colchicine and glucocorticoids.”
A total of 53 percent of the patients assigned to apremilast group and 22 percent of the placebo group were free from oral ulcers at week 12, and improvements were observed in overall disease activity and patient-reported outcomes. Decreases in the ulcer number and the associated pain were maintained through week 28 with continued treatment.
“Nausea, diarrhea, upper respiratory tract infection, headache, upper abdominal pain, and vomiting occurred in a higher percentage of patients receiving apremilast than of those receiving placebo,” the authors wrote.
The authors suggested that trials using an active comparator and longer follow-up are required to explore the longer-term efficacy and safety of apremilast.
REFERENCE
Gülen Hatemi, Alfred Mahr, Yoshiaki Ishigatsubo, et al. “Trial of Apremilast for Oral Ulcers in Behçet’s Syndrome.” NEJM. November 14, 2019. DOI: 10.1056/NEJMoa1816594




