Article
Apremilast Safely Treats Patients with Psoriasis, Psoriatic Arthritis in Real-World Setting
Author(s):
A long-term, post-authorization safety study of adverse events was performed for patients with PsO and/or PsA exposed to apremilast.
No new adverse events or safety signals were reported in a study evaluating the long-term safety of apremilast-treated patients with psoriasis (PsO) and psoriatic arthritis (PsA) in a real-world setting. Results were comparable to those of previous clinical trials, according to a study published in Springer.1
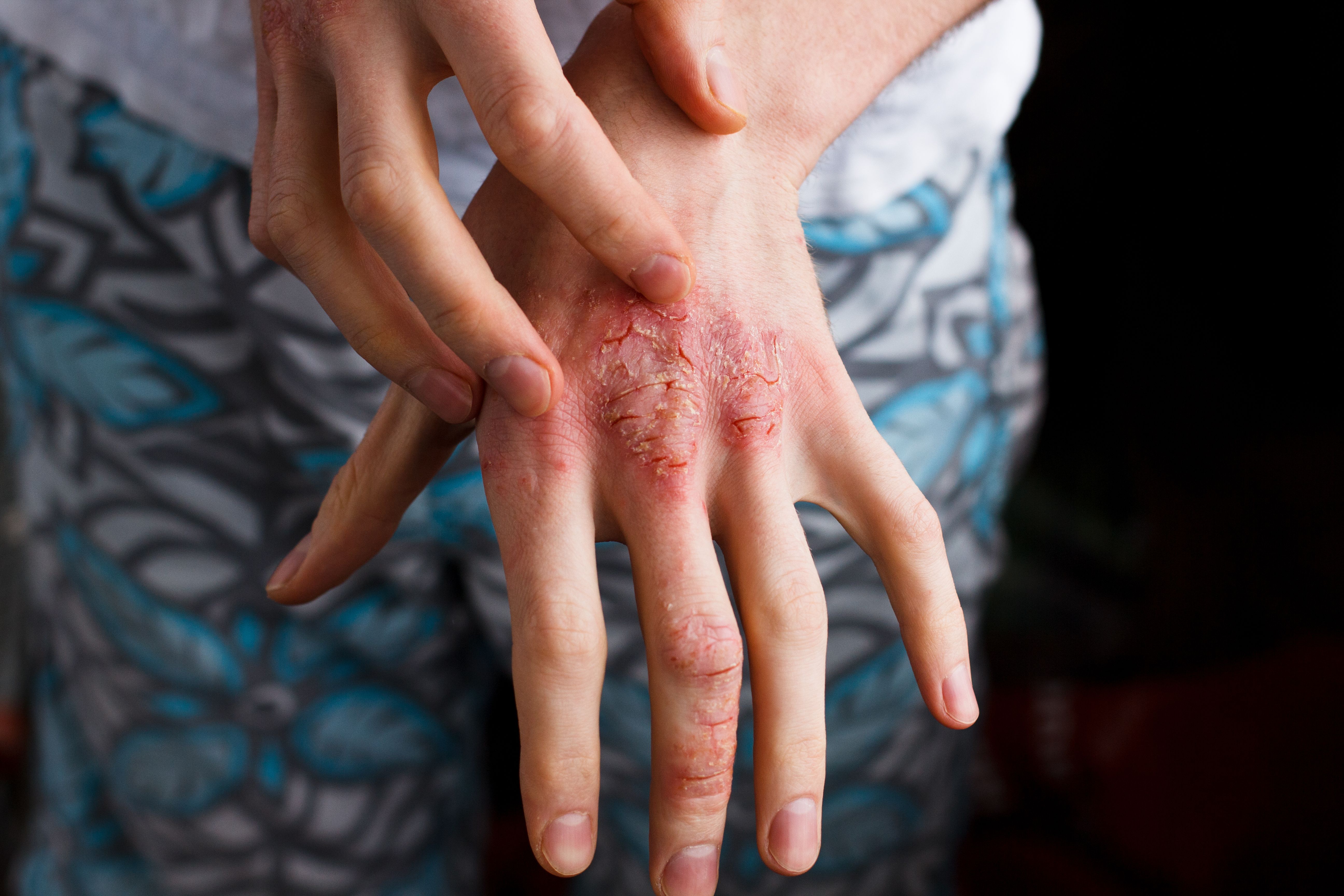
“In 2015, apremilast was approved in Europe for the treatment of moderate-to-severe plaque PsO in adults who do not respond to, have a contraindication to, or are intolerant of other systemic therapy, or for the treatment of active PsA, alone or in combination with disease-modifying antirheumatic drugs (DMARDs),” investigators explained. “Following the European Medicine Agency’s approval of apremilast, the European Medicines Agency requested a post-authorization safety study to provide long-term surveillance of adverse events of special interest (AESIs) in patients with PsO and/or PsA exposed to apremilast.”
A 5-year cohort study was performed between January 2015 and June 2020 utilizing patients with PsO and PsA registered in the United Kingdom (UK) Clinical Practice Research Datalink (CPRD) GOLD. Adverse events of special interest were analyzed for patients receiving apremilast as well as 3 non-apremilast cohorts: oral only, injectable only, and oral and injectable PsO and PsA treatments. Non-apremilast drugs included DMARDs, oral or injectable steroids, non-tumor necrosis factor inhibitor biologics, and azathioprine. All patients were matched for age, sex, calendar time, and year of record start with up to 10 patients in each non-apremilast cohort. Eligible patients had a diagnosis of PsO and/or PsA and 1 or more prescriptions for a non-apremilast comparison treatment within 6 months or any time after an apremilast-exposed patient’s first apremilast prescription.
The apremilast cohort included 341 patients, while the non-apremilast cohorts consisted of 4981 patients. Comorbidities were similar across all groups; however, apremilast-exposed patients had a lower prevalence of anxiety and depression. There were no cases of hematologic malignancy, non-melanoma skin malignancy, treated anxiety or depression, suicidal behaviors, or vasculitis in those receiving apremilast during the follow-up period. Incidence rates of all-cause mortality, solid malignancies, tachyarrhythmias, and major adverse cardiac events were similar across all arms.
The incidence rate of both opportunistic and serious infections were similar between the apremilast cohort and the oral and injectable non-apremilast cohorts (61 [40-102]; 50 [42-60]; and 57 [47-69], respectively). However, the incidence rates were lower in the injectable treatment-only group (20 [10-41]).
The small number of apremilast-exposed patients and potential exposure misclassification limited the study. Additionally, the PsO and PsA treatments in the CPRD data is incomplete. Infusion drugs, which are generally received in outpatient clinics, as well as biologics and other oral specialty treatments prescribed by specialists, are not included in the general practitioners (GP) record. There is also a possibility that patients receiving apremilast from a specialist were not included in the record. Lastly, patients in the apremilast cohort may have entered the study further into the disease pathway when compared with non-exposed apremilast patients, as the PsA criteria is less strict, and physicians may experience more delays in diagnosing PsA earlier.
“These results provide evidence that the long-term safety profile of apremilast in PsO and PsA in a real-world setting is comparable to that reported in clinical trials and other real-world publications,” investigators concluded.
Reference:
Persson R, Cordey M, Paris M, Jick S. Safety of Apremilast in Patients with Psoriasis and Psoriatic Arthritis: Findings from the UK Clinical Practice Research Datalink [published online ahead of print, 2022 Sep 23]. Drug Saf. 2022;1-9. doi:10.1007/s40264-022-01235-7




