Article
Are Silicone Breast Implants Safe?
Author(s):
Though silicone breast implants only received approval from the US Food and Drug Administration (FDA) in 2006 after a 14-year hiatus, new data is still surfacing and being analyzed.

Silicone breast implants have long been believed to play a role in some incidents of breast cancer, as well as the degeneration of patients’ surrounding connective tissue. Though silicone breast implants only received approval from the US Food and Drug Administration (FDA) in 2006 after a 14-year hiatus, new data is still surfacing and being analyzed. So, where does the truth lie and speculation begin?
In June 2011, the FDA released a report compiling the results of all published studies dealing with the long-term safety of silicone breast implants. At the time, the FDA had approved 2 types of silicone breast implants for cosmetic purposes: Allergan’s Natrelle Silicone Gel-Filled Breast Implants and Mentor’s MemoryGel Silicone Gel-Filled Breast Implants. Both of those products were only approved after extensive FDA testing with patients monitored for their long-term safety.
Over an 8- to 10-year period, the most common side effects of both implants included capsular contracture, reoperation, implant removal, and rupture. Allergan’s patients also faced issues such as breast asymmetry, scarring, and pain, while Mentor’s patients experienced problems like changes in nipple and breast sensation.
This chart gives a high-level snapshot of the complications and adverse reactions from both silicone implant manufacturers:
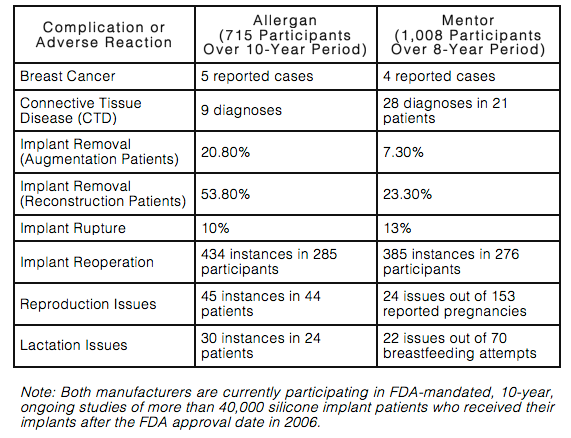
Women who obtain silicone breast implants must realize that their risk of complications increases over time. The longer a woman has her implants, the greater her chances of needing additional surgery to modify, remove, or replace them. However, this fact is not widely known by the many women who come to me seeking consultation for breast augmentation or reconstruction.
As for the speculation that this cosmetic procedure heightens a woman’s risk of developing breast cancer, women who receive silicone breast implants are not found to be at an increased risk. In fact, some studies suggest that these women may even be at an average or lower risk, with a risk reduction of 10-50%. Nevertheless, the FDA officially concluded that these studies have not been performed on a large enough scale to completely rule out a link to more serious diseases.
For the early detection of breast cancer, women must recognize that their implants may obscure traditional mammograms. Breast implants make it difficult for the machine to detect small amounts of abnormal breast tissues, which are the early signs of potential cancer. However, additional X-ray images with implant displacement views can be obtained for a more complete screening.
Among my post-surgery patients, I strongly recommend scheduling routine follow-up visits, which include periodic MRI exams to detect implant “silent ruptures” or tears in the implant that causes the solution to leak without any symptoms in the patient. First and foremost, patients need to notify me immediately if they develop any unusual signs or symptoms including pain, asymmetry, hardness, or swelling of the breast.
Based on the evidence, the FDA believes that silicone breast implants have a reasonable assurance of safety and effectiveness when used as labeled by a licensed professional. Ultimately, I believe it is the patient’s responsibility to be informed of the benefits and risks of any type of breast implant, as is true with any type of surgery.
Robert T. Grant, MD, MSc, FACS, is Chief of the combined Divisions of Plastic Surgery at New York-Presbyterian Hospital-Columbia University Medical Center and New York-Presbyterian Hospital-Weill Cornell Medical Center. He is also Associate Clinical Professor of Surgery in the College of Physicians and Surgeons at Columbia University and Adjunct Associate Professor of Clinical Surgery at Weill Cornell Medical College. For more information about Dr. Grant or to contact him, visit his website at www.robertgrantmd.com.





