Article
Arthritis in a patient with severe psoriasis: Narrowing the differential
An 84-year-old woman presented with pain and swelling in her knees and hands. She had a history of severe psoriasis and osteoarthritis. How can you make a differential diagnosis when OA may coexist with rheumatoid arthritis, psoriatic arthritis, OA, and crystal arthritis? Read on to find out.
An 84-year-old woman presented with a 1-week history of pain and swelling in her knees and hands. She had a past medical history significant for severe psoriasis and osteoarthritis (OA). The differential diagnosis included rheumatoid arthritis (RA), psoriatic arthritis (PsA), OA, and crystal arthritis, but we did not rule out any diagnoses because various arthritides may coexist.
A complete history and examination are important, even when the diagnosis seems clear, because making a correct diagnosis is paramount to avoiding exposure of the patient to unnecessary adverse effects of treatment aimed at other conditions. In this article, we present a case report describing our patient and the process we went through in narrowing the differential and determining appropriate treatment.
Case presentation
The patient reported morning stiffness and swelling lasting more than 60 minutes. On examination, there was swelling, warmth, and tenderness at the proximal interphalangeal joints, metacarpophalangeal (MCP) joints, wrists, and knees. Heberden and Bouchard nodes were present. Skin findings included diffuse scaly, erythematous plaques consistent with severe psoriasis.
Therapy with prednisone, 60 mg/d, had been started by the hospital team 3 days before they consulted rheumatology, and the patient was already experiencing some relief. She did not have "sausage digits" or nail pitting, but her arthritis was symmetrical. She was taking only topical creams for her psoriasis, for which the diagnosis had been made more than 20 years earlier.
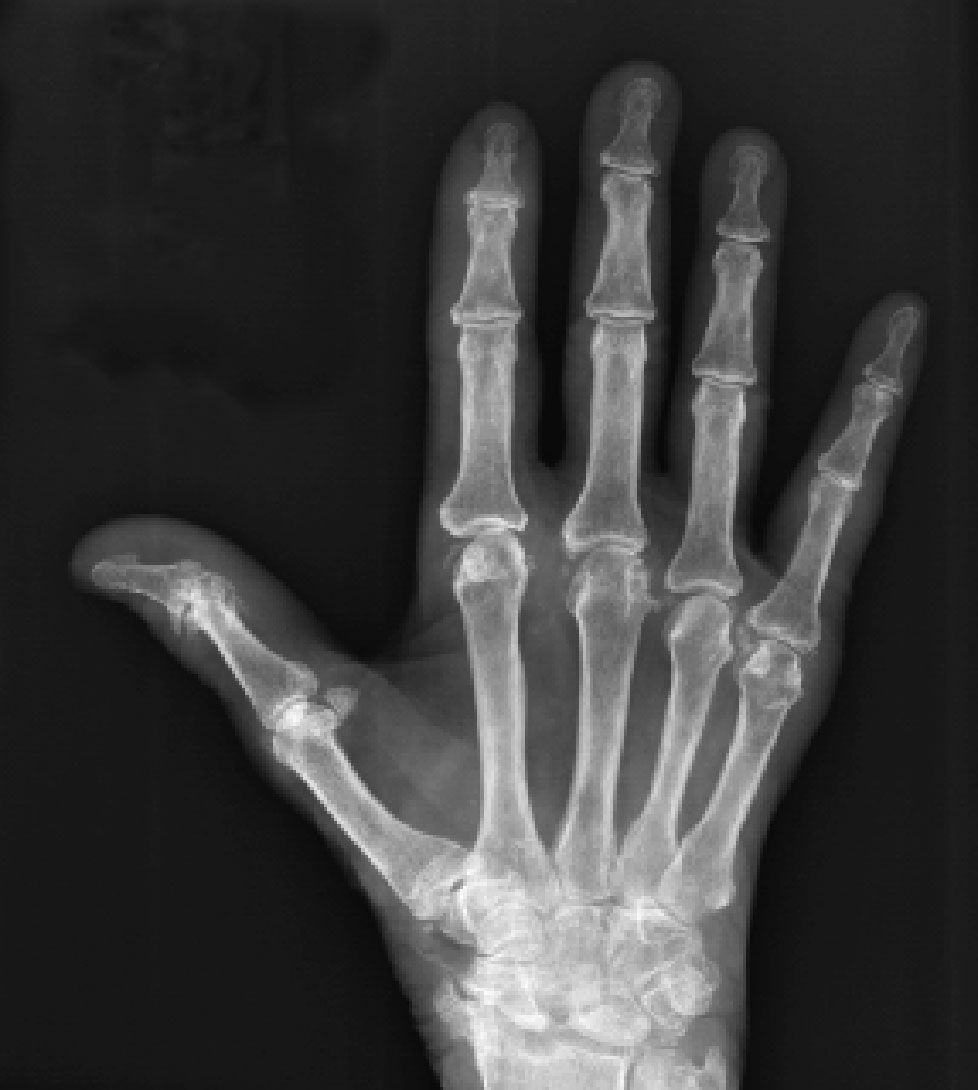
Figure 1 – A radiograph of our patient's hand shows chondrocalcinosis in the triangular fibrocartilage as well as in many of the intercarpal and metacarpophalangeal joints. Severe degenerative changes have occurred throughout the carpus, with marked scapholunate disassociation.
Considering the patient's symmetrical arthritis involving small and large joints with severe psoriasis, our top 2 considerations in the differential diagnosis were psoriasis and RA. Rheumatoid factor (RF) and cyclic citrullinated peptide IgG antibody test results were negative. X-ray films of the patient's hands revealed chondrocalcinosis in the triangular fibrocartilage, as well as in many of the MCP joints (Figure 1). In addition, there were severe degenerative changes throughout the carpus with marked scapholunate disassociation and narrowing of the triscaphe joint (between the scaphoid, trapezium, and trapezoid). On the basis of the imaging findings, a diagnosis of calcium pyrophosphate dihydrate (CPPD) deposition disease was made.
X-ray films of the knees revealed chondrocalcinosis in the fibrocartilage (menisci) and in the hyaline cartilage outlining the femoral condyles and tibial plateaus (Figure 2). This finding is unique to CPPD deposition disease, but the patient also had secondary degenerative joint disease.
Further testing showed a white blood cell count of 8.5 × 103/μL (normal range, 4.5 to 11 × 103/μL); hemoglobin level, 10.7 g/dL (normal, 12 to 16 g/dL); hematocrit level, 37.7% (normal, 37% to 47%); platelet count, 240,000/μL (normal, 140,000 to 440,000/μL); calcium level, 8.3 mg/dL (normal, 8.6 to 10 mg/dL); phosphate level, 2.9 mg/dL (normal, 2.5 to 4.5 mg/dL); magnesium level, 1.8 mEq/L (normal, 1.8 to 2.6 mEq/L); C-reactive protein level, 89 mg/L (normal, lower than 5); erythrocyte sedimentation rate, 127 mm/h (normal, 0 to 20 mm/h); uric acid level, 7.4 mg/dL (normal, 3 to 8.2 mg/dL); vitamin D level, 8.8 ng/mL (normal, 15.9 to 55.6 ng/mL); and parathyroid hormone (PTH) level, 154 pg/mL (normal, 16 to 65 pg/mL). Because the PTH level remained elevated on repeated testing, an endocrinology consultation was requested; the endocrinologist made an additional diagnosis of vitamin D deficiency.
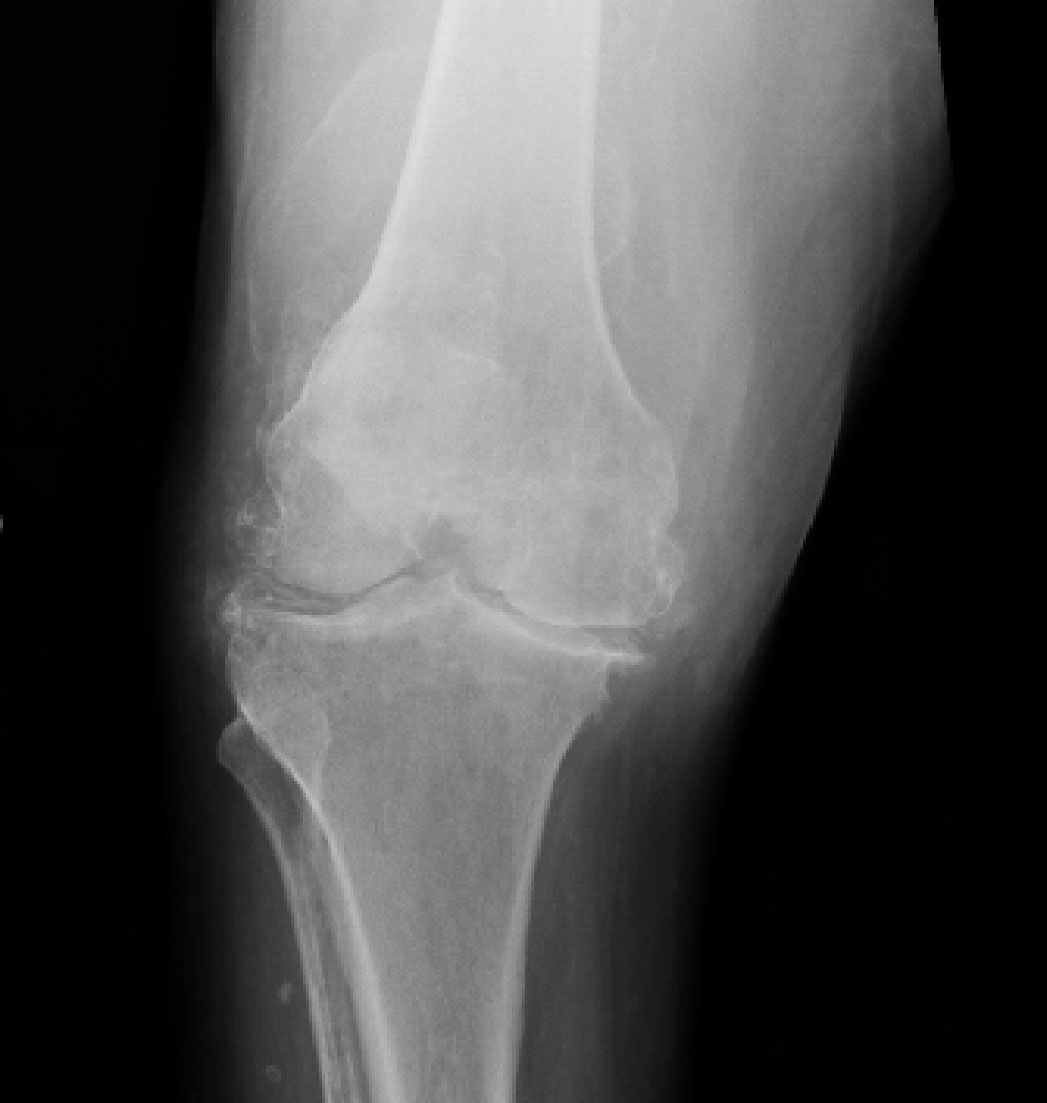
Figure 2 – A knee radiograph shows chondrocalcinosis in both the fibrocartilage (menisci) and hyaline cartilage outlining the femoral condyles and tibial plateaus. Severe secondary degenerative joint disease also is present in our patient.
Discussion
The patient had symmetrical polyarthritis with a long history of psoriasis and severe arthritis. The
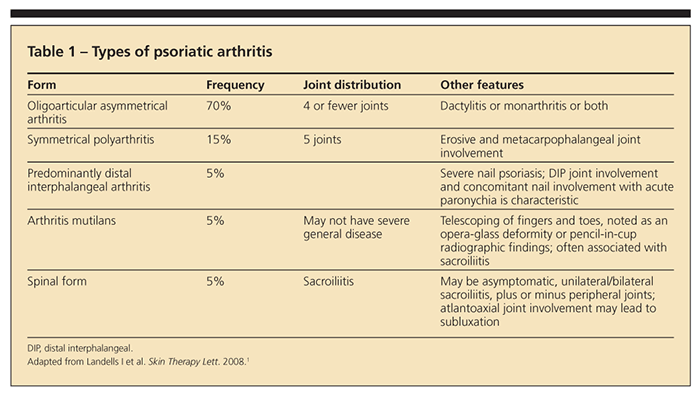
presence of psoriatic dermal signs is not pathognomic for PsA, which may have several manifestations (Table 1).1
PsA is a type of arthritic inflammation that occurs in about 30% of patients with psoriasis.2 Symmetrical PsA accounts for about 15% of cases of PsA. Wilson and associates3 observed that nail dystrophy, scalp lesions, and intergluteal and perianal psoriasis are psoriatic features associated with a higher probability of PsA.
In 2005, the ClASsification of Psoriatic ARthritis (CASPAR) study group put forth a set of criteria for the diagnosis of PsA.4 To meet the CASPAR criteria, a patient must have inflammatory articular disease (joint, spine, or entheseal) with 3 or more points from 5 categories (Table 2).
Current psoriasis is assigned a score of 2; all other features have a score of 1. The CASPAR criteria
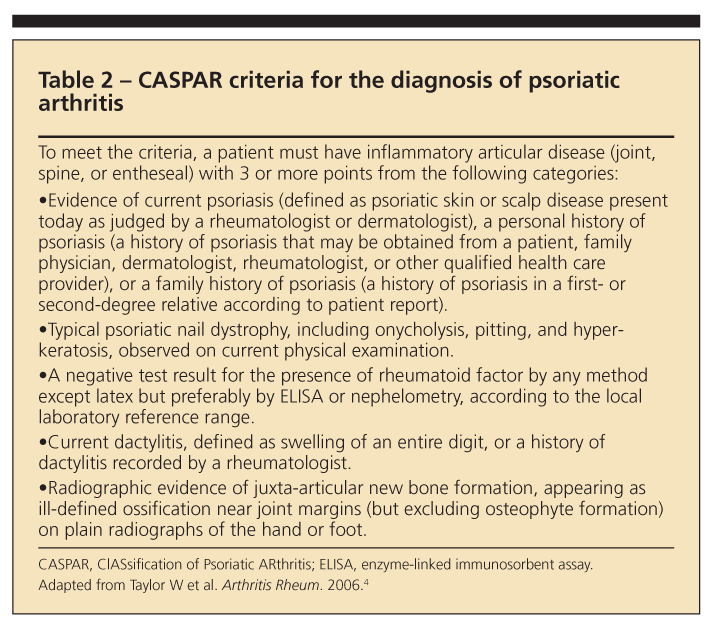
have a specificity of 98.7% and a sensitivity of 91.4%.4 Our patient had a score of 3 points, making a diagnosis of PsA a possibility.
Regardless of the criteria, many patients may have PsA without skin disease; isolated nail psoriasis is observed in 3% of the patients with psoriasis.5 Note that most criteria were intended as clinical research inclusion criteria and not diagnostic criteria.
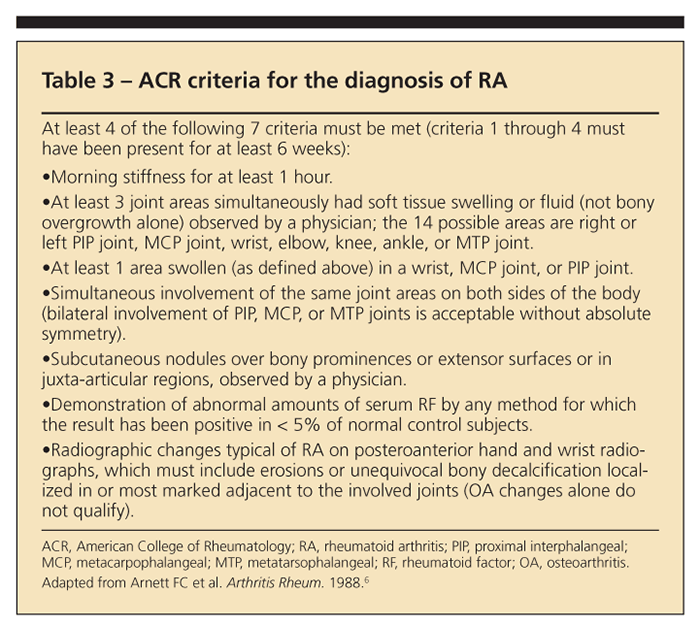
RA would be a possibility in the differential of symmetrical PsA. However, the American College of Rheumatology (ACR) criteria, in which at least 4 of 7 findings must be met, should be considered (Table 3).6 Our patient met 3 of the 7 ACR criteria. Therefore, she did not qualify for a diagnosis of RA. The patient would have been characterized by this diagnosis if her RF had been positive, which is not uncommon in her age-group.
Crystal-induced arthritis usually is monarticular. However, polyarticular acute flares are not uncommon and many joints may be involved simultaneously or in rapid succession.
Our patient had an inflammatory arthritis. Therefore, crystal arthritis-including gout-was included in the differential diagnosis. According to the American Rheumatism Association, a patient has gout if at least 7 of 13 criteria are present (Table 4).7
An arthrocentesis would have been useful, because the diagnosis of gout includes the presence of intracellular negatively birefringent monosodium urate crystals, but was not performed for our patient. However, the patient's x-ray films lacked the classic findings of gout and the history was not suggestive of the condition.
In addition, although elevated serum uric acid levels are an important risk factor for gout, they neither confirm nor exclude the disorder. During acute attacks of gout, serum uric acid levels may be normal; conversely, many persons with hyperuricemia do not have gout. In fact, psoriasis with high tissue nucleic acid turnover also may lead to mild to moderate

hyperuricemia.8
Chondrocalcinosis-a pathological and radiographic term denoting stippled calcification of cartilage within joints, including both hyaline articular cartilage and fibrocartilage-usually affects middle-aged and older persons. A review of the literature suggests that this nomenclature involves an area of confusion. Chondrocalcinosis is not synonymous with CPPD crystals. The term also may refer to dicalcium phosphate dihydrate crystals or calcium hydroxyapatite crystal deposition disease (HADD). However, CPPD crystal deposition accounts for about 95% of chondrocalcinosis cases.9
The occurrence of intra-articular chondrocalcinosis also may be associated with OA.10 However, plain x-ray films are not very sensitive for the diagnosis of chondrocalcinosis; in fact, Fisseler-Eckhoff and Mller11 showed that a radiographic diagnosis of chondrocalcinosis is made in only about 40% of patients who have pathologically proven CPPD crystal deposition.
CPPD disease is characterized radiologically by the presence of chondrocalcinosis or involvement of an unusual joint or both. The classical radiological features in the wrist include calcification of the triangular fibrocartilage between the distal ulna and the carpal bones. There also may be solitary narrowing of the radiocarpal joint, which may progress to narrowing of the triscaphe joint. Chondrocalcinosis also may progress and be seen in the ligaments between the various carpal bones, particularly in the scapholunate and lunotriquetral joints but also on the metacarpal heads and in the interphalangeal joints.
In the knee, apart from the presence of chondrocalcinosis in both the fibrocartilage and hyaline cartilage (a finding unique to CPPD), the most characteristic finding is solitary narrowing of the patellofemoral joint without visible narrowing of the lateral or medial compartment. Chondrocalcinosis may be seen in other joints, including the hips, shoulders, and symphysis pubis. Note that chondrocalcinosis is a radiographic diagnosis and a radiological entity that may occur with or without clinical manifestations.
The most likely diagnosis in our patient, without our having performed aspiration, was pseudogout. The presence of CPPD crystals accounts for most cases of crystal-induced arthritis, apart from gout. The clinical presentation of CPPD crystal deposition is highly variable. Five clinical patterns have been described: asymptomatic, pseudogout, pseudorheumatoid, pseudo-osteoarthritis, and pseudo-neuropathic joint disease pattern; 50% of cases are idiopathic.
CPPD crystal deposition is thought to be associated with hypomagnesemia, hypophosphatasia, hyperparathyroidism, and hemochromatosis, but this possible association is controversial. A proposed mechanism of secondary CPPD crystal deposition in hyperparathyroidism is the associated hypercalcemia. The cause of CPPD crystal deposition is not known, but 2 mechanisms have been proposed, overproduction or decreased removal of CPPD crystals from cartilage and abnormality of the underlying cartilage collagen.
Increasing age has been proposed to predispose persons to crystal deposition by changing the concentration of proteoglycan and inhibitory factors and increasing inorganic phosphate turnover. The diagnosis of CPPD crystal deposition disease is based largely on the presence of positively birefringent intracellular CPPD crystals, but insufficient fluid in our patient after she had received high doses of prednisone for 3 days resulted in joint aspiration not being performed. Crystal arthritis was not our top diagnosis.
Treatment of patients with CPPD crystal deposition is geared mainly toward symptomatic relief. Aspiration of large effusions, NSAIDs, intra-articular corticosteroid injection or oral doses of corticosteroids, and low-dose colchicine may be used. There is no medical treatment for calcium deposits or polyarticular and progressive degenerative changes. Successful management of hyperparathyroidism or hemochromatosis has not been shown to reverse chondrocalcinosis, but managing the disease correctly prevents future flares and worsening of the disease condition.
HADD is characterized by the deposition of calcium hydroxyapatite crystals in para-articular soft tissues, resulting in tendinitis and bursitis; the cause is unknown. Hydroxyapatite crystals have a characteristic amorphous paste-like appearance on x-ray films.
HADD was a possibility in our patient. However, it more typically affects middle-aged persons and men and has a different joint distribution (ie, shoulders), although calcifications may be found in every joint and multiple deposits are common.11 HADD has been found to be associated with chronic renal failure, collagen vascular disease, and trauma.12
Our patient may qualify for systemic management of psoriasis, which may include methotrexate or biologic agents. However, managing her psoriasis may not help her arthritis. We lowered our patient's dosage of prednisone to 5 mg 3 times a day and then tapered it. We started therapy with colchicine, 0.6 mg twice daily, and supplemental vitamin D. The patient's symptoms have since improved.
References:
References1. Landells I, MacCallum C, Khraishi M. The role of the dermatologist in identification and treatment of the early stages of psoriatic arthritis. Skin Therapy Lett. 2008;13:4-7.
2. Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol. 2003;4:441-447.
3. Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61:233-239.
4. Taylor W, Gladman D, Helliwell P, et al; CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665-2673.
5. Kaur I, Saraswat A, Kumar B. Nail changes in psoriasis: a study of 167 patients. Int J Dermatol. 2001;40:601-603.
6. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315-324.
7. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
8. Pittman JR, Bross MH. Diagnosis and management of gout. Am Fam Physician. 1999;59:1799-1806, 1810.
9. McCarty DJ Jr, Hogan JM, Gatter RA, Grossman M. Studies on pathological calcifications in human cartilage, I: prevalence and types of crystal deposits in the menisci of two hundred fifteen cadavers. J Bone Joint Surg. 1966;48A:309-325.
10. Reuge L, Van Linthoudt D, Gerster JC. Local deposition of calcium pyrophosphate crystals in evolution of knee osteoarthritis. Clin Rheumatol. 2001;20:428-431.
11. Fisseler-Eckhoff A, Müller KM. Arthroscopy and chondrocalcinosis. Arthroscopy. 1992;8:98-104.
12. Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics. 1990;10:1031-1048.




