News
Article
Biologics for Psoriasis Less Effective Among Individuals with Specific Clinical Characteristics
Author(s):
These data resulted from a new meta-analysis in which biologic therapies were shown to be less effective among psoriasis patients with clinical characteristics such as age and BMI.
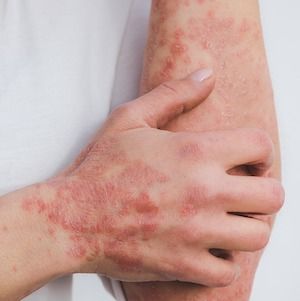
Biologic responses are not as strong among individuals with psoriasis who are older, smoke cigarettes, have a smoking history, have prior exposure to biologic treatments, or have a higher body mass index (BMI), according to recent findings, though the impact of these clinical characteristics on treatment response is unclear.1
These conclusions were the results of a new meta-analysis which was led by Gustav Hjort, MS, from the department of dermatology and allergy at Copenhagen University Hospital – Herlev and Gentofte in Denmark. Hjort et al. noted that prior research indicating certain clinical characteristics are negatively associated with biologic efficacy, though such associations were not shown to exist in other studies.2
The investigators sought to assess the potential of a link between these types of clinical characteristics and the effectiveness of biologic therapy among patients with psoriasis.
“To date and to our knowledge, no pooled analysis has examined the associations between clinical characteristics and the effectiveness of biologics in patients with psoriasis,” Hjort and colleagues wrote. “Therefore, we conducted a meta-analysis on this topic.”
Background and Methods
The investigators implemented a search strategy which involved using terms connected to psoriasis, disease characteristics, or predictors, in addition to terms used for biologics, the drugs’ mechanisms of action, and generic names. The investigators carried out a comprehensive search of 3 specific databases, namely Web of Science, PubMed, and Embase, up to April 2022.
For research to qualify for this meta-analysis, studies needed to be written in English, contain original content, and report on any links observed between clinical characteristics and patients achievement of a Psoriasis Area and Severity Index (PASI) score improvement of 75% (PASI 75) or 90% (PASI 90) at the 12, 26, or 52-week mark.
PASI75 or 90 among psoriasis patients would be assessed among those treated with biologic therapies at approved dose levels. The research team’s main outcome of interest had been PASI 90 at 26 weeks specifically, while PASI 75 and PASI 90 at other points in time were considered to be secondary outcomes of interest.
The team implemented the Rayyan software for their blinded screening process, later assigning 2 specific authors to screen titles as well as abstracts. These authors would get rid of duplicates and identify research meeting the analysis’s criteria for inclusion or that had ambiguous titles/abstracts.
The investigators resolved any discrepancies through discussion, with only the most recent and comprehensive publications used when questions arose. The team’s full-text screening of the research involved reviewers gathering data on such topics as the first authors, titles, years of publication, country, study database/department(s), design of study, duration, criteria for inclusion, average ages, sample size, patient gender ratio, average weight, average BMI, baseline PASI scores, and information on missing data.
Clinical characteristics related to treatment response were extracted, as were details on biologic therapies and other topics. The investigators would include clinical characteristics only assuming they had been evaluated within at least 3 studies that had the same outcome and design.
Findings
Overall, the research team found 40 studies for their meta-analyses, the sum of which which involved 21,438 participants. The team reported that factors such as previous treatment with biologic therapies (odds ratio [OR], 0.44; 95% CI, 0.29-0.67), older age (OR, 0.99; 95% CI, 0.98-1.00), and history of smoking (OR, 0.81; 95% CI, 0.67-0.98) were found to be linked with lower likelihood of PASI 90 achievement at the 6-month mark in observational studies.
Additionally, they found that individuals who were currently smoking (OR, 0.78; 95% CI, 0.66-0.91) or had higher BMI (OR, 0.96; 95% CI, 0.94-0.99) were also shown to have less of a chance at achieving PASI 90.
The investigators added that, within randomized controlled trials (RCTs), there had been only a BMI of 30 or higher which demonstrated a negative association with response to biologic treatment. This was specifically PASI 90 at 3 months (OR, 0.57; 95% CI, 0.48-0.66).
“However, there is a lack of evidence regarding whether these effects, as well as others, vary by biologic therapy,” they wrote. “Future studies on this topic may guide clinicians to stratify treatment based on patient characteristics.”
References
- Hjort G, Schwarz CW, Skov L, Loft N. Clinical Characteristics Associated With Response to Biologics in the Treatment of Psoriasis: A Meta-analysis. JAMA Dermatol. Published online June 18, 2024. doi:10.1001/jamadermatol.2024.1677.
- Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41(10):917-925. doi:10.1007/s40261-021-01080-z.





