Article
Defining Osteoarthritis: What It Is, and What It Is Not
Synovial inflammation in osteoarthritis (OA) is secondary to mechanical damage to the articular cartilage and bone. OA often is said to be a disease of weight-bearing joints, but it is more appropriate to consider them as load-bearing joints. OA is the failure of an organ, the synovial joint.
Because of its implications for prevention and treatment, how a clinician views osteoarthritis (OA) matters. In most cases, the real problem for the patient is not OA but painful OA. Synovial inflammation in OA results from mechanical damage to the articular cartilage and bone; therefore, it stands to reason that NSAIDs, although they may be symptomatically effective, cannot arrest the underlying process.
Efforts to develop disease-modifying OA drugs (formerly called chondroprotective drugs and now structure-modifying OA drugs) to interrupt or reverse the underlying pathogenetic processes have not succeeded. Foremost among the reasons, in this author’s opinion, is a failure to appreciate sufficiently that common, garden-variety OA-from its earliest stages and in whichever joint it occurs-is a mechanically induced and mechanically driven disease caused by an excessive level of mechanical stress (force/unit area) or by aberrant stress of a physiological magnitude acting on habitually loaded areas of the joint, resulting in local biochemically mediated damage.
In this 3-part article, I review the pathogenesis, diagnosis, and management of OA. This first part offers a contemporary, operational, evidence-based definition of the disease that has evolved from growing knowledge of its causes.1,2 In the second and third parts, to appear in upcoming issues of this journal, I will review the clinical aspects of OA, including diagnosis and diagnostic pitfalls, and approaches to treatment.
FACTS RELEVANT
TO A DEFINITION
Of note, OA does not develop in all abnormal joints. Among patients who in childhood had hip dysplasia, slipped capital femoral epiphysis, or Legg-Calv-Perthes disease, conditions well known to predispose persons to hip OA, the frequency of OA at a 30-year follow-up evaluation was only 60% to 70%, not 100%.3 Although all patients had hips that were at increased risk for OA, the likelihood that OA would develop in a predisposed person presumably was affected by the severity of the structural abnormality, the amount of unprotected loading, and the adequacy of the mechanisms that normally protect joints from excessive mechanical stress.
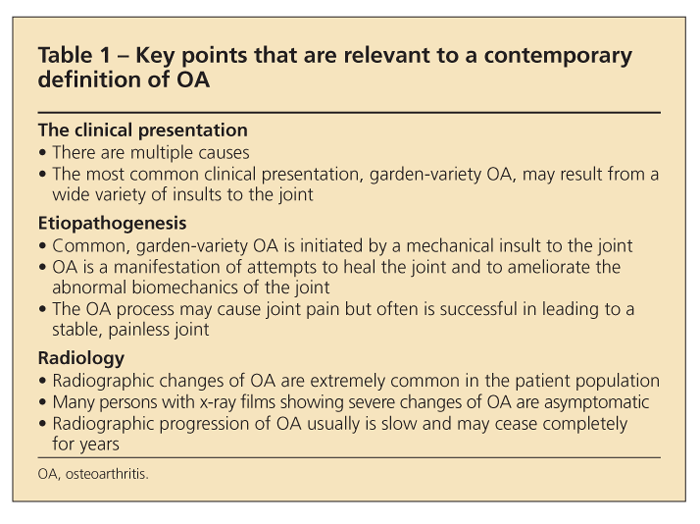
Table 1 lists several facts that are relevant to a contemporary definition of OA. Although OA often is said to be a disease of weight-bearing joints (eg, the hip and knee) because most of the load on a synovial joint stems not from the ponderal mass but rather from the strength of contraction of the periarticular muscles, considering the joints as load-bearing joints is more appropriate.
The load across the small joints of the hand that is created by contraction of the powerful flexor digitorum profundus muscle may be roughly comparable to that across the knee or hip. The prevalence of OA is greater in distal interphalangeal joints than in proximal interphalangeal joints or metacarpophalangeal joints, possibly because the load-bearing surface available in the former is only about one-fourth to one-half as large as that in the latter and the underlying cushion of metaphyseal trabecular bone is thinner. Implicated as the cause of OA is not simply the force but also the concentration of that force across the joint and the rate of joint loading.
OA is organ failure of the
synovial joint
OA is the failure of an organ, the synovial joint. Just as the heart can fail because of a primary problem in the endocardium, myocardium, or epicardium, the joint can fail because of a primary problem in any of its tissues-ligaments, meniscus, subchondral bone, periarticular muscle, synovium, nerves, or articular cartilage-and OA can originate in any of them. Therefore, it is to be expected that there are many causes of OA, and there are.
For this reason, OA has no common pathophysiological pathway but only a final common end stage. The inflammatory changes in OA are secondary and are caused by particulate and soluble breakdown products of cartilage and bone.
Not just a“cartilage disease”
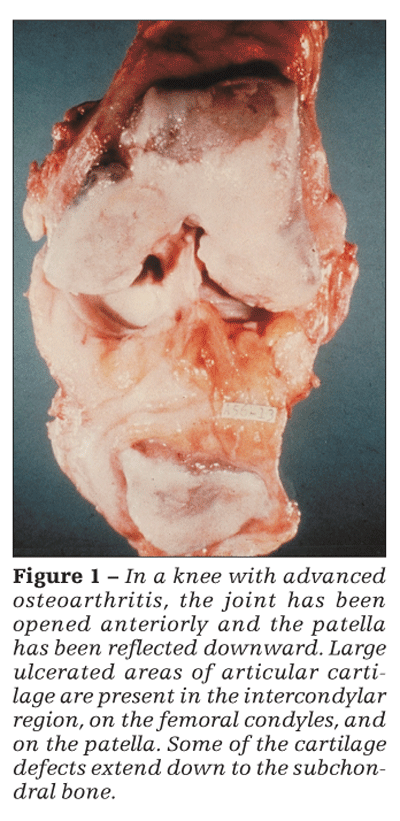
Although articular cartilage involvement is prominent in OA (Figure 1), by no means is OA merely a cartilage disease. This concept is reinforced by the generally poor correlation between the severity of cartilage loss (as reflected, eg, by radiographic joint-space narrowing) and the severity of symptoms. Also, among patients with knee OA who underwent an osteotomy (to relieve unacceptably high intra-articular stress), improvement 2 years later was unrelated to whether the articular surface was now hyaline cartilage or fibrocartilage or was denuded relative to that seen at a baseline arthroscopy-ie, the cartilage histology did not seem to affect the patient’s clinical status.4
Chondrocentric definitions that focus on the loss of articular cartilage have not helped understanding of the etiopathogenesis of OA, a concern different from that of the pathogenesis of articular cartilage damage caused by cytokines, tissue-degrading enzymes, and toxic oxygen radicals. Also, most current definitions of OA do not recognize that OA reflects a repair process intended to contain joint damage caused by a local mechanical problem. Under the appropriate conditions (correction of abnormal stress, joint motion, and establishment of a source of cells), joints with OA can heal-with structural and symptomatic improvement.5
Not a degenerative joint disease
OA often is viewed incorrectly as a condition that, once it becomes symptomatic, follows an inexorably progressive and downhill course, much like Alzheimer disease. Physicians who convey this view to their patients do them a disservice. Many patients with OA can be made better. In fact, many will do well-perhaps better-without a physician’s intrusion.
OA should not be considered a degenerative joint disease because the cartilage and bone cells essentially are normal and, if high levels of intra-articular stress are reduced, retain the capacity to restore the damaged tissue to normal. Beyond the inherent scientific inaccuracy, consider the pessimism, sense of futility, and nihilism generated in patients with painful OA whose physicians tell them that they have degenerative joint disease.
A disorder called OA that is not OA
Confusion often results from insufficient recognition that not all articular cartilage damage progresses to OA. Several investigators have established that OA involves loss of articular cartilage, in particular, in the habitually load-bearing areas of the joint. In contrast, articular cartilage damage in habitually unloaded areas of the joint (a true degenerative change caused by the absence of loading) does not progress.6
Unless chondromalacia occurs in a joint that also has severe OA involvement whose subchondral bone has remodeled in response to abnormal stresses, it is an asymptomatic cartilage change that will not lead to full-thickness cartilage loss. The bony changes that are of essential pathogenetic importance in OA do not occur in habitually unloaded areas, and chondromalacia generally is associated with osteopenia of the underlying bone rather than with bony thickening.
With the advent of arthroscopy, many surgeons have been able to monitor articular cartilage lesions in their patients over time. They have observed, for example, that most patellar medial facet cartilage lesions do not progress. Indeed, vertical cracks in the articular surface generally do not progress. Deep horizontal splits in the cartilage are required if the surface is to undergo fragmentation; cartilage that fibrillates only vertically will remain in place.
Differentiating these areas of nonprogressive cartilage degeneration from OA is important. They are asymptomatic and do not lead to joint-space narrowing or bony changes of OA on radiography. On MRI, however, they are often misinterpreted as OA; arthroscopists, mistakenly thinking that the superficial cartilage changes are OA, may damage the joint unnecessarily.
CAN WE WEAR OUT OUR JOINTS?Repetitive impulsive loading (RIL)
After normal joints were oscillated ex vivo for up to 500 hours under loads as high as 500 kg, they showed no signs of cartilage damage.7 Attempts to wear away the cartilage by oscillation under increasingly higher loads eventually resulted in fracture of the underlying bone but left the cartilage intact.
However, when RIL (with rapid ramping up of the applied load, eg, only 50 milliseconds from onset to peak) was added to oscillation to roughly simulate the physiological conditions of joint loading in gait and activities of daily living, the articular cartilage was rapidly worn off the joint.7 Thickening of the subchondral bone preceded breakdown of the cartilage. In vivo confirmation of the potential of impulsive loading to cause joint damage is provided by studies in adult rabbits, in which the forces produced by hopping were simulated by brief periods of RIL.8
Under these conditions, numerous trabecular microfractures occurred, with stiffening of the underlying subchondral bone. This was followed by progressive damage to the articular cartilage, with deep fibrillation and horizontal splitting. Importantly, when loads of the same or greater magnitude were applied gradually (eg, 500 milliseconds, onset to peak), they had no effect.
Because the quadriceps muscle is the major antigravity muscle of the lower extremity and serves as a brake on the pendular action of the lower limb during ambulation, minimizing the forces generated with heel strike, it plays a major role in protecting against mechanical damage to the knee. During gait, healthy subjects decelerate their leg before heel strike can create an impulsive load. Among healthy subjects who had no force-transient profile during gait, the load rate increased more than 2-fold (to about 150 × body weight/s) after a femoral nerve block to temporarily paralyze the quadriceps muscle.9
This suggests that a heel strike transient may be caused by the failure of quadriceps contraction to decelerate the lower extremity in the swing phase of gait. In healthy subjects, minor incoordination in muscle recruitment that results in failure to decelerate the leg may generate impulsive forces as high as 65 × body weight/s at heel strike.9
A comparison of young adult subjects whose knee x-ray film results were normal but who had intermittent activity-related knee pain with age-matched, asymptomatic, radiographically normal controls showed that the 2 groups were similar with respect to gait patterns, walking speeds, terminal stance phase knee flexion, maximum (peak) swing phase angular velocity, and overall shape of the ground reaction.10 However, the groups differed markedly over the few milliseconds surrounding heel strike.
The downward velocity of the ankle just before heel strike and the impact at heel strike were greater in the group that had knee pain than in the controls. Immediately after heel strike, the follow-through of the leg was more violent in the subjects who had knee pain, with larger peak axial and angular accelerations of the leg that were reflected in a more rapid increase of the ground reaction force. The mean rate of loading of the knee (velocity at heel strike adjusted for body weight) among those who loaded their knee impulsively was significantly higher than that of those who did not.
These changes, which were not visible to the naked eye, replicated those seen in experimental animals that were subjected to RIL8 and consistently resulted in OA. About one-third of the subjects exhibited microincoordination during gait and impulsively loaded their knees repetitively while they walked on a level surface. Radin and associates10 referred to these subjects as “microklutzes.” In young adults, microklutziness is associated with knee pain. A limp and reduced walking speed decrease RIL.
Peak adduction moment (PAM)
The PAM reflects the magnitude of the intrinsic compressive load on the medial tibiofemoral compartment in stance. A considerable body of work related to the PAM adds to the evidence that biomechanical abnormalities drive the etiopathogenesis of common, garden-variety knee OA.11
Varus-valgus alignment is a key determinant of the PAM. Varus malalignment further increases the medial compartment load during gait; valgus malalignment increases stress in the lateral compartment. Malalignment may be a consequence of knee OA but also may result from genetic or developmental factors or previous trauma. The PAM predicts radiographic progression in persons with medial compartment OA12 and development of knee pain in asymptomatic older persons.13
In cross-sectional studies, the PAM has been shown to be significantly greater in patients with medial compartment knee OA than in controls14 (and in subjects with more severe OA than in those with less severe disease15). In subjects who had relatively mild radiographic knee OA, medial compartment loads were significantly higher among those who had knee pain than those who were asymptomatic.
A greater degree of toe-out during walking, which reduces the PAM, diminished the risk of radiographic progression in subjects with medial compartment knee OA. Use of lateral wedge orthoses for more than a year resulted in significant and persistent decreases in the PAM compared with use of neutral orthoses. Also, use of lateral wedge orthoses recently was found to slow the progression of medial compartment joint-space narrowing over 3 years relative to that seen with neutral orthoses,16 suggesting that structural damage, and not only symptoms, improves as a result of improved biomechanics.
PROTECTING AGAINST MECHANICAL DAMAGE
When a normal joint is in the unloaded state, the opposing surfaces are incongruent. With loading, the cartilage and bone on both sides of the joint space deform, maximizing the contact area and thereby minimizing stress within the cartilage.17
With aging, the congruity of joints increases, rendering them less flexible under load. Although the prevalence of OA clearly increases with age and age is the major risk factor for OA, nearly one-third of knee joints of human tissue donors who were in their seventh through ninth decade and had no history of arthritis showed no evidence of OA.18 The increased prevalence of OA in older persons most likely reflects a gradual accumulation of microdamage to the joint over a lifetime rather than a direct consequence of the aging of joint tissues.
During normal walking, 3 to 4 times body weight is transmitted through the knee. During a deep knee bend, the patellofemoral joint is subjected to a load as great as 9 to 10 times body weight. Although the bulk properties of articular cartilage would make it an excellent shock absorber, in most joints it is too thin to serve as much of a shock-absorbing structure. Therefore, adaptive mechanisms are needed to protect joints from these physiological loads.
Viscoelasticity is important
Because articular cartilage is viscoelastic (has a fluid/matrix composite that permits movement of interstitial fluid through its matrix), when it is loaded, fluid flows away from the load and weeps from the surface at the periphery of the loaded area. This hydrostatic compression, which is essential to the function of articular cartilage, transmits the load to the underlying subchondral bone, sparing the cartilage matrix and cells from damage.2
If deformation of the articular cartilage with loading is restricted so that the cartilage cannot conform to the load completely, the size of the contact area becomes restricted and high stresses are generated within the cartilage. If the cartilage is thinned, as in OA, the process is exacerbated.
Like articular cartilage, normal subchondral bone is viscoelastic; the fluid phase is bone marrow. Bone deforms less, or becomes stiffer, when the load is applied rapidly than when loading is more gradual. As a consequence, the chondroprotective effect of the shock absorbing of the subchondral bone has limits. This is why joint damage caused by excessive loading is related to the rate of loading as well as to the magnitude of the load. On the basis of the results of the animal studies and observations in humans described herein, and our understanding of the structure, composition, and material properties of articular cartilage, it is likely that rapid RIL does not allow sufficient time for interstitial fluid to flow and absorb the energy transmitted and thereby protect the cartilage matrix and cells.
Articular cartilage viscoelasticity helps protect the cartilage matrix. However, most of the shock absorption is provided by subchondral, metaphyseal, and diaphyseal bone and, as noted below, by the periarticular soft tissues, especially muscle.
Periarticular muscles: active shock absorption
Intermittent loading is the normal mode of joint loading in most activities of daily living. Consider the loading of the lower extremity in walking or running or of the upper extremity in hammering a nail, playing tennis, or shoveling snow. That diarthrodial joints are so highly susceptible to damage from RIL suggests that musculoskeletal shock absorbers are needed. Periarticular soft tissues (eg, capsule, ligaments, synovium) and bone have significant force-attenuating properties, but articular cartilage and synovial fluid have little effect.
The most important of the shock-absorbing mechanisms that protect the joints involve timely muscle contraction.1 Muscles not only move a joint, they function as large rubber bands. When a muscle that is under slight stretch is subjected to greater stretch by movement of the joint, it can absorb a great deal of energy.
Most of the muscle activity generated during ambulation is used not to propel the body but to absorb energy to decelerate the body. For example, a person who jumps off a ledge or table normally lands on his or her toes, comes down on his heels, and then straightens his flexed knees and hips. During this coordinated action, the person’s muscles perform negative work, ie, they absorb energy: as the ankles are dorsiflexed, the calf muscles are stretched, and as the knees and hips are straightened, the hamstrings are stretched. The amount of energy absorbed as a result of this stretching is great.2
Consider what happens when a person descends a flight of stairs, misjudges the distance to the next step, and abruptly skips that step. Because the muscles are not prepared to accommodate the load, a sharp jolt is felt.
Because about 75 milliseconds is required to prepare a neuromuscular reflex to handle an impact load, an unexpected fall of a very brief distance (eg, about 1 inch) does not afford sufficient time to bring protective muscular reflexes into play and the load is transmitted to the cartilage and bone. A fall from a greater height affords time to activate the appropriate reflexes, so that flexing the joint and, on landing, lengthening of the muscles that span the joint absorbs the energy of the impact and protects the cartilage and bone.
Conditions such as sarcopenia, aging, disuse atrophy, neuropathies, and diminished proprioceptive acuity may impair this protective mechanism. With RIL, insufficient time is available for the neuromuscular system to protect the joint.2
Quadriceps weakness with knee OA
In patients with knee OA, quadriceps muscle weakness is common. Although such weakness generally has been ascribed to disuse atrophy that developed because the patient minimized use of the painful limb, it also may exist in persons with knee OA who have no history of joint pain and in whom quadriceps muscle mass is not diminished but is normal or even increased (as a result of obesity).
Longitudinal studies have suggested that quadriceps weakness not only may result from the pain of knee OA but also may be a risk factor for knee OA.19 Among women, but not among men, with radiographically normal knees at the initial examination in whom definite radiographic OA had developed 30 months later, baseline knee extensor strength was significantly lower than that among women in whom radiographic OA did not develop. Each 10 lb/ft increase in extensor strength was associated with 20% and 29% decreases in the odds of the development of radiographic and symptomatic knee OA, respectively. A relatively small increase in strength (about 20% of the mean for men and 25% for women) was predicted to result in a 20% to 30% reduction in the odds of incident knee OA.
More recently, thigh strength was found to predict incident symptomatic disease in subjects who had radiographic OA at baseline.20 Also, in a study of subjects who had knee OA or known risk factors for the condition, quadriceps weakness in women, but not in men, was associated with an increased risk of radiographic narrowing of the tibiofemoral or patellofemoral joint or both.21
Subchondral bone and passive shock absorption
The subchondral bone is of major pathogenetic importance in OA. In early OA, the subchondral trabecular bone in the diseased joint stiffens. In experimental OA and in human joints, microfractures that heal with microcallus are evident in the subchondral trabeculae.
Marked stasis of medullary blood flow, venous engorgement, and intraosseous hypertension all have been documented in patients with painful hip OA.22 Radin23 related these hemodynamic abnormalities to the consequences of repair of microfractures caused by RIL, which results in an increase in the volume of subchondral trabecular bone.
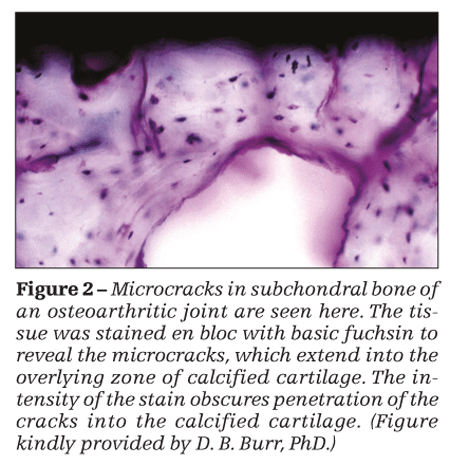
Microfractures represent damage of the trabeculae below the subchondral plate. In contrast to microfractures, interstitial microcracks are found within the subchondral plate and in the zone of calcified cartilage (Figure 2) and are a manifestation of damage of the subchondral plate.
High-impact loads cause microcracks in the calcified cartilage. Similarly, low-magnitude RIL causes microcracks in the calcified cartilage and damage to the hyaline articular cartilage and subchondral bone.
The significance of microcracks in OA lies in their stimulation of focal remodeling, which may reduce the concentration of excessive or aberrant peak dynamic loads. In addition, they can create channels for capillary ingrowth that may transmit biologic mediators between the marrow cavity and articular cartilage. Because microcracks are associated with reactivation of the secondary center of ossification, duplication of the tidemark and advance of the latter toward the joint space, and endochondal ossification, they lead to thinning of the hyaline articular cartilage from below and therefore play a significant role in the etiopathogenesis of the structural damage of OA.
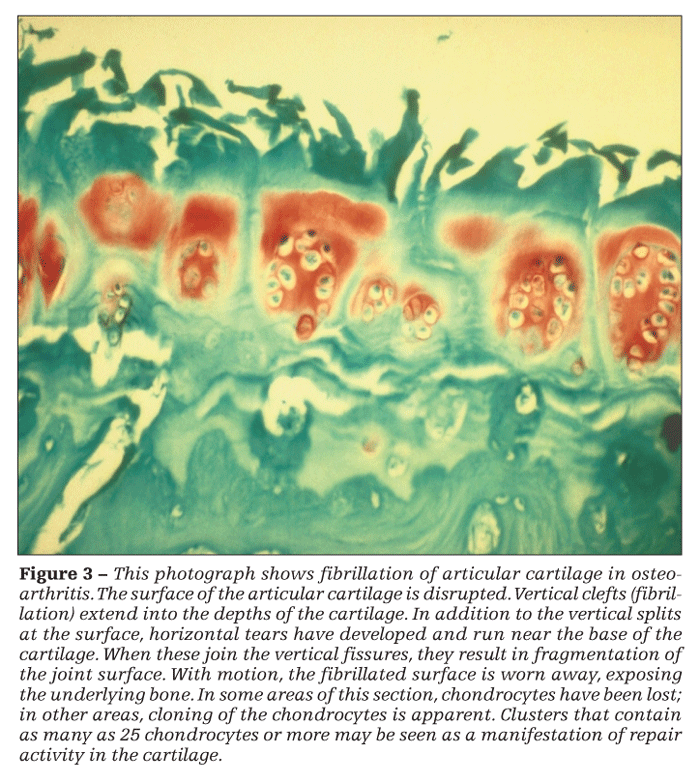
Thinning of the cartilage increases shear stresses in the tissue, leading to the propagation of horizontal clefts (Figure 3). Where these clefts join vertical fibrillations, shards of cartilage are broken off the surface. They may be recovered in the synovial fluid or found embedded in the synovium, where they incite an inflammatory response.
Clinical relevance
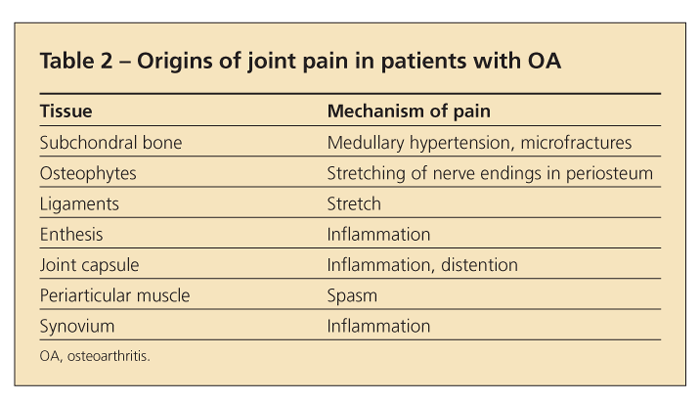
Because articular cartilage is aneural, the joint pain in OA must arise from other structures (Table 2). In some cases, the pain may result from stretching of nerve endings in the periosteum covering osteophytes. In others, it may arise from synovitis or from microfractures in subchondral bone or may reflect bone angina, caused by the distortion of medullary blood flow by the thickened subchondral trabeculae (which may appear as subchondral sclerosis on x-ray films).
The tidemark, as noted above, may increase the intraosseous pressure and can cause severe intraosseous stasis, with a decrease in oxygen tension and increases in carbon dioxide tension and
lactate concentration. This hemodynamic abnormality is reflected in a prolonged emptying time after intraosseous injection of radiopaque contrast material into the femoral neck. Hunter and colleagues24 confirmed the link between elevated intraosseous venous pressures and bone marrow lesions (BMLs).
Like synovitis, medullary hypertension is an important cause of OA pain. Normalization of the hemodynamic changes by osteotomy promptly alleviates joint pain in patients with OA. Follow-up of those patients who have undergone a hip or knee osteotomy that has been planned and executed carefully so as to relieve the stress concentration on the load-bearing surface of the joint has shown that the procedure can produce not only long-term pain relief and functional improvement but also reversal of joint-space narrowing and subchondral sclerosis.
The association between joint pain and BMLs in patients with knee OA also should be noted. BMLs, frequently seen in fat-suppressed T2-weighted MRI scans of knees with OA as focal areas of increased signal in the subchondral marrow, are consistent with the marrow lesions that McAlindon and coworkers25 described in 1991 and showed to be strongly associated with isotope retention on a bone scan. Although BMLs mistakenly have been called bone marrow “edema,” they represent foci of fibrosis, osteonecrosis, and bone remodeling. They are not specific for OA and may be seen with insufficiency fractures, osteonecrosis, and a variety of other conditions.
In patients with knee OA, a very strong correlation exists between isotope retention in subchondral bone on scintigraphy and subchondral sclerosis on radiography. Dieppe and associates26 showed that bone scans have predictive value for radiographic progression of knee OA. Among scans that did not show focal areas of isotope retention, no progression was noted over a 5-year follow-up period; in contrast, progression was seen in about 50% of patients with OA who had a “hot” knee scan at baseline.
A preliminary report of a cross-sectional study of subjects with symptomatic OA described a strong association between degradation of the meniscus and BMLs.27 Large BMLs in the medial tibiofemoral compartment were observed only if there was evidence of damage of the medial meniscus. Although a longitudinal study will be needed to establish causality, the authors suggested that BMLs are a consequence of impact forces in a knee with aberrant load distribution or instability resulting from meniscus damage. As further indication of the strong association between BMLs and mechanical stress, BMLs are much more prevalent in the medial tibiofemoral compartment of varus knees than they are in knees with neutral or valgus alignment (about 74% vs 16%). In valgus knees, they tend to localize to the lateral compartment.28
The efficacy of osteotomy and joint distraction
The evidence indicating that a joint with OA may be improved by normalizing excessive intra-articular stress with osteotomy is reinforced by evidence indicating that unloading a joint with OA with distraction also may result in long-term symptomatic and structural improvement. Lafeber and colleagues29 emphasized the utility of unloading the OA joint with osteotomy, distraction, footwear, or bracing.
Use of joint distraction to manage OA is based on the premise that stiffening of the sclerotic subchondral bone plays a role in the development and progression of damage in the overlying articular cartilage. Observations that significant decreases in pain and functional improvement that were seen after joint distraction in patients with advanced posttraumatic ankle OA were associated with a reduction in the density of subchondral bone of the talus and tibia suggest that a more physiological distribution of mechanical stress by the less dense bone permitted repair of the damaged cartilage.30
Osteotomy has been largely superceded by arthroplasty (amputation of the joint) as a surgical treatment for patients with painful OA. In the hands of most orthopedic surgeons, the latter is a more reliable procedure and, for the patient, is followed by a much more rapid return to load-bearing activity. However, the earlier work on osteotomy and the more recent studies cited above, which show that mechanical interventions that decrease loading of the joint with OA can improve symptoms and structure, serve as proof of the etiopathogenetic importance of abnormal joint mechanics in OA.
CONCLUSIONS
That the patient who presents with common, garden-variety OA represents the failed repair of mechanically induced joint damage has obvious implications for treatment. Unless the abnormal intra-articular stress that got the joint into trouble in the first place is corrected, interruption or reversal of the progression of structural damage with a chondroprotective drug or biologic agent is unlikely. On the other hand, if the abnormal stress is corrected, pharmacological intervention may be superfluous or useful only as an adjunct.
In efforts to find new OA therapies, a search for the genes that control the shape of joints or neuromuscular coordination or otherwise influence joint loading may be more fruitful than a search for genes that regulate the synthesis or breakdown of articular cartilage. A recent post hoc analysis of data from the trial of doxycycline in knee OA, in which varus angulation negated the significant structure-modifying benefit of the drug,31 supports this view.
The data on the magnitude of the PAM and rates of loading await reconciliation. Meanwhile, it seems abundantly clear that mechanical abnormalities play a central role in the etiopathogenesis of the structural abnormalities and symptoms of knee OA and that if the excessive load borne by a joint with OA is redistributed, the joint can heal.
References:
References
1. Brandt KD, Dieppe P, Radin EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am. 2008;34:531-559.
2. Brandt KD, Dieppe P, Radin EL. Commentary: is it useful to subset “primary” osteoarthritis? A critique based on evidence regarding the etiopathogenesis of osteoarthritis. Semin Arthritis Rheum. 2009;39:81-95.
3. Weinstein SL. Bristol-Myers Squibb/Zimmer award for distinguished achievement in orthopaedic research. Long-term follow-up of pediatric orthopaedic conditions: natural history and outcomes of treatment. J Bone Joint Surg. 2000;82A:980-990.
4. Bergenudd H, Johnell O, Redlund-Johnell I, Lohmander LS. The articular cartilage after osteotomy for medial gonarthrosis: biopsies after 2 years in 19 cases. Acta Orthop Scand. 1992;63:413-416.
5. Radin EL, Burr DB. Hypothesis: joints can heal. Semin Arthritis Rheum. 1984;13:293-302.
6. Hirsch C. The pathogenesis of chondromalacia of the patella: a physical, histologic and chemical study. Acta Chir Scand. 1944;90(suppl 83).
7. Simon SR, Radin EL, Paul IL, Rose RM. The response of joints to impact loading, II: in vivo behavior of subchondral bone. J Biomech. 1972;5:267-272.
8. Radin EL, Boyd RD, Martin RB, et al. Mechanical factors influencing cartilage damage. In: Peyron JG, ed. Osteoarthritis: Current Clinical and Fundamental Problems. 2nd ed. Paris: Geigy; 1985:90-99.
9. Radin EL, Whittle MW, Yang KH, et al. The heelstrike transient, its relationship with the angular velocity of the shank, and the effects of quadriceps paralysis. In: Lantz SA, King AI, eds. Advances in Bioengineering. New York: American Society of Mechanical Engineering; 1986:121-123.
10. Radin EL, Yang KH, Riegger C, et al. Relationship between lower limb dynamics and knee joint pain [published correction appears in J Orthop Res. 1991;9:776]. J Orthop Res. 1991;9:398-405.
11. Andriacchi TP. Dynamics of knee alignment. Orthop Clin North Am. 1994;25:395-403.
12. Miyazaki T, Wada M, Kawahara H, et al. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617-622.
13. Amin S, Luepongsak N, McGibbon CA, et al. Knee adduction moment and development of chronic knee pain in elders. Arthritis Rheum. 2004;51:371-376.
14. Baliunas AJ, Hurwitz DE, Ryals AB, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:573-579.
15. Mündermann A, Dyrby CO, Hurwitz DE, et al. Potential strategies to reduce medial compartment loading in patients with knee osteoarthritis of varying severity: reduced walking speed [published correction appears in Arthritis Rheum. 2004;50:4073]. Arthritis Rheum. 2004;50:1172-1178.
16. Gocker B, Demirag MD, Block JA. Lateral wedge orthotics delay progression of joint space narrowing in patients with medial knee osteoarthritis. Arthritis Rheum. 2008;58(suppl):S241.
17. Bullough P, Goodfellow J, O’Connor J. The relationship between degenerative changes and load-bearing in the human hip. J Bone Joint Surg. 1973;55B:746-758.
18. Loeser RF, Shakoor N. Aging or osteoarthritis: which is the problem? Rheum Dis Clin North Am. 2003;29:653-673.
19. Slemenda C, Heilman DK, Brandt KD, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951-1959.
20. Segal NA, Torner JC, Felson D, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61:1210-1217.
21. Segal NA, Glass NA, Torner J, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage. 2010;18:769-775.
22. Arnoldi CC, Linderholm H, Müssbichler H. Venous engorgement and intraosseous hypertension in osteoarthritis of the hip. J Bone Joint Surg. 1972;54B:409-421.
23. Radin EL. Osteoarthrosis-the orthopedic surgeon’s perspective. Acta Orthop Scand Suppl. 1995;266:6-9.
24. Hunter DJ, Niu J, Zhang Y, et al. Altered perfusion and venous hypertension is present in regions of bone affected by BMLs in knee OA. Osteoarthritis Cartilage. 2007;15(suppl C):C171.
25. McAlindon TE, Watt I, McCrae F, et al. Magnetic resonance imaging in osteoarthritis of the knee: correlation with radiographic and scintigraphic findings. Ann Rheum Dis. 1991;50:14-19.
26. Dieppe P, Cushnaghan J, Young P, kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52:557-563.
27. Lo G, Hunter D, Nevitt M, et al. Strong association of meniscal maceration and bone marrow lesions in osteoarthritis. Arthritis Rheum. 2007;56(suppl):S125.
28. Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139(5, pt 1):330-336.
29. Lafeber FP, Intema F, Van Roermund PM, Marijnissen AC. Unloading joints to treat osteoarthritis, including joint distraction. Curr Opin Rheumatol. 2006;18:519-525.
30. Intema F, van Roermund PM, Castelein RM, et al. Joint distraction in the treatment of knee osteoarthritis: the first clinical results. Osteoarthritis Cartilage. 2007;15(suppl C):C234.
31. Mazzuca SA, Brandt KD, Chakr R, Lane KA. Varus malalignment negates the structure-modifying benefits of doxycycline in obese women with knee osteoarthritis. Osteoarthritis Cartilage. 2010;18:1008-1011.




