Article
Dexcom Announces Global Launch of Dexcom G7 Glucose Monitoring System
Courtesy: Dexcom
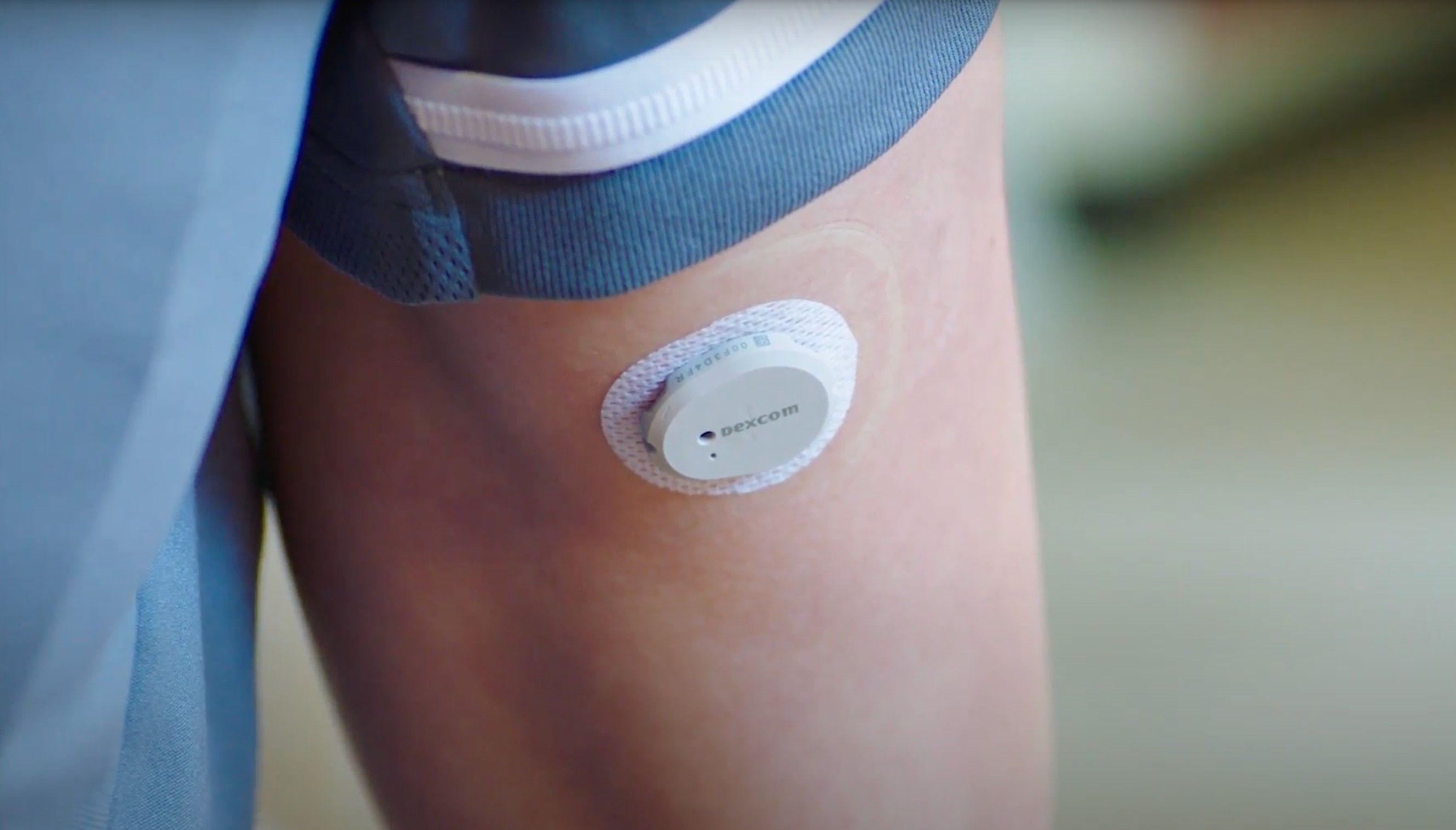
The Dexcom G7 Continuous Glucose Monitoring (CGM) System rollout is officially underway with Dexcom announcing its availability in 5 countries in an announcement on October 4.
Now available for people with diabetes aged 2 years and older in the United Kingdom, Ireland, Germany, Austria, and Hong Kong, the company’s announcement, which was made during a global streaming event, also noted plans for a rollout of the Dexcom G7 CGM system in New Zealand and South Africa in the coming weeks.
“Since Dexcom G6 came to market, we’ve eliminated over 15 billion fingerpricks and helped improve the lives of more than 1.25 million people with diabetes around the world. We’re thrilled to begin the global rollout of Dexcom G7, our next-generation CGM technology, that vastly improves upon what everyone loves about G6,” said Kevin Sayer, chairman, president and CEO of Dexcom.
Billed as the company’s smallest, most-intuitive real-time CGM, the next-generation Dexcom G7 CGM system boasts several notable improvements compared to the previous Dexcom G6 CGM system, including being 60% smaller, a 30-minute sensor warm-up, and a 12-hour grace period to replace finished sensors. In addition to the system’s new features, the company also introduced a redesigned mobile app.
In their announcement, DexCom highlighted the Dexcom G7 CGM system will continue to corporate popular features from the Dexcom G6 CGM system, including delivery of real-time glucose readings sent directly to compatible devices, ability to share glucose data with up to 10 individuals, and not requiring finger sticks, scanning, or calibration. Dexcom also noted plans for a future software release that would allow for the system to send information directly to an Apple Watch.
“Dexcom CGM has become the gold standard of care for diabetes and bringing our technology to more and more people around the world continues to be a top priority,” Sayer added. “With this initial launch behind us, we look forward to introducing G7 in additional markets and bringing this life-changing technology to as many people as we can.”
According to a Q2 2022 earnings call transcript on SeekingAlpha, Sayer noted a limited US rollout was targeted for late 2022 with a large commercial launch planned in Q1 of 2023. This delay is because of a change to the G7 software based on feedback from the US Food and Drug Administration.




