Article
Diagnosing fibromyalgia: Moving away from tender points
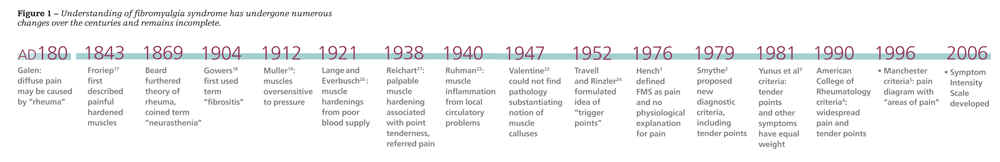
Fibromyalgia syndrome (FMS) is not a novel entity-there has been interest in unexplained pain syndromes since antiquity (Figure 1). The earliest research focused on the symptom of “muscle hardenings,” which may be the equivalent of musculoskeletal symptoms that patients with FMS describe today.
Fibromyalgia syndrome (FMS) is not a novel entity-there has been interest in unexplained pain syndromes since antiquity (Figure 1). The earliest research focused on the symptom of “muscle hardenings,” which may be the equivalent of musculoskeletal symptoms that patients with FMS describe today. More recently, the concept of FMS diagnosis has undergone several changes, including a shift in focus from tender point evaluation to identifying “areas of pain” and from assessment of specific symptoms to looking at the “whole patient.”
As in all other illnesses, clinicians should actively seek a diagnosis of recognition to facili- tate better overall management. A multimodal approach to therapy should be executed in parallel rather than in tandem to achieve optimal benefit.
In this article, we review early and recent developments in the diagnosis of FMS. We also discuss key treatment issues: the importance of patient education, the role of aerobic exercise, and the use of therapeutic agents in optimizing sleep and in managing depression and pain.
EVOLUTION OF THE DIAGNOSTIC CONCEPT
Initial criteria
In 1976, Hench1 clinically defined FMS on the basis of 2 criteria, pain and no physiological explanation for pain. Since this initial description, FMS has evolved into a diagnosis of inclusion rather than exclusion. In other words, physicians should make a diagnosis of FMS on the basis of its clinical presentation rather than on a battery of tests that are not useful in the diagnosis of this condition and may be potentially misleading because of the detection of incidental findings. Because FMS does not offer immunity to other diseases, however, a reasonable clinically indicated workup is not unjustified.
In 1979, Smythe2 proposed diagnostic criteria for FMS as 12 of 14 tender anatomical points (tender with application of 4 kg of pressure), diffuse pain of at least 3 months’ duration, disturbed sleep, skin roll tenderness at the upper trapezius border, and normal laboratory test results. In 1981, Yunus and associates3 included diffuse pain of 3 months’ duration, lack of other obvious causes, and 5 of 40 tender points along with 10 other minor criteria. These criteria gave tender points and other FMS symptoms and signs equal weight.
In 1990, the American College of Rheumatology (ACR) set criteria for FMS on the basis of a study of 293 patients deemed to have FMS compared with 265 control patients matched for age, sex, and rheumatologic diagnoses.4 The combination of criteria of widespread pain of at least 3 months’ duration with 11 of 18 tender points provided a sensitivity of 88% and specificity of 81%. On the basis of the ACR criteria, 19% of patients with 11 tender points did not have FMS. This criteria set ignored the myriad other symptoms and signs that are part of FMS, including fatigue; cognitive problems; sleep difficulties; feeling unrefreshed in the morning; hypersensitivity to light, odor, and sound; and generalized weakness.
Tender points revisited
Since the ACR definition was set, the concept of tender points has been revisited, with increasing counterargument for their use in the diagnosis of FMS (Figure 2). Tenderness over certain anatomical areas may be elicited in normal persons with application of moderate pressure. However, tender points probably are more useful as a measure of “severity” of symptoms that a patient with FMS experiences. Therefore, they may be used as a barometer of psychological distress but probably should not be used as prima facie evidence of FMS.
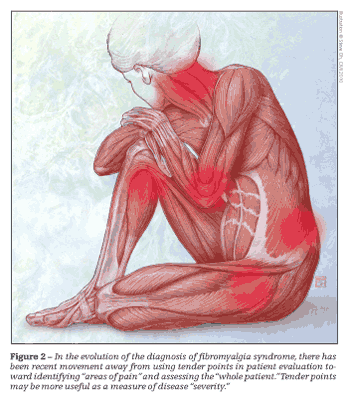
Multiple studies have lent support to the concept of relying less heavily on tender points in the diagnosis of FMS. The Manchester criteria used a pain diagram in which patients could indicate areas of pain rather than be limited in their choice to predefined tender points.5 The move to expand the diagnostic process of FMS to include other common symptoms of FMS rather than limit it to the narrow realm of pain also was obvious in the London Fibromyalgia Epidemiology Study Screening Questionnaire, the first test to assess both pain and fatigue as an epidemiological screen for FMS.6 In 1999, White and colleagues7 emphasized the coexistence of fatigue with tender points in patients with FMS. This concept further evolved into a formal measurement tool, the Symptom Intensity Scale (SIS).
Objective assessment tool
The SIS is a comprehensive, objective tool for assessing pain and fatigue in patients with FMS (Figure 3). This scale helps in the diagnosis of FMS as well as in serial monitoring akin to serially measuring markers of inflammation in systemic inflammatory autoimmune disorders. Patients can fill out the scores at each visit, providing themselves and the treating physician with an objective measure rather than the vague “I am having a good (or bad) day.” In addition, the reward of seeing improvement in their SIS measures encourages patients to adhere to the advice that the physician offers during the office visit.
Wolfe8 developed the SIS on the basis of a survey mailed to 12,799 patients who had various rheumatologic diagnoses, including rheumatoid arthritis (RA), osteoarthritis, and FMS. The survey consisted of a pain scale that queried about 38 articular and nonarticular areas and a 10-cm Fatigue Visual Analogue Scale (FVAS).
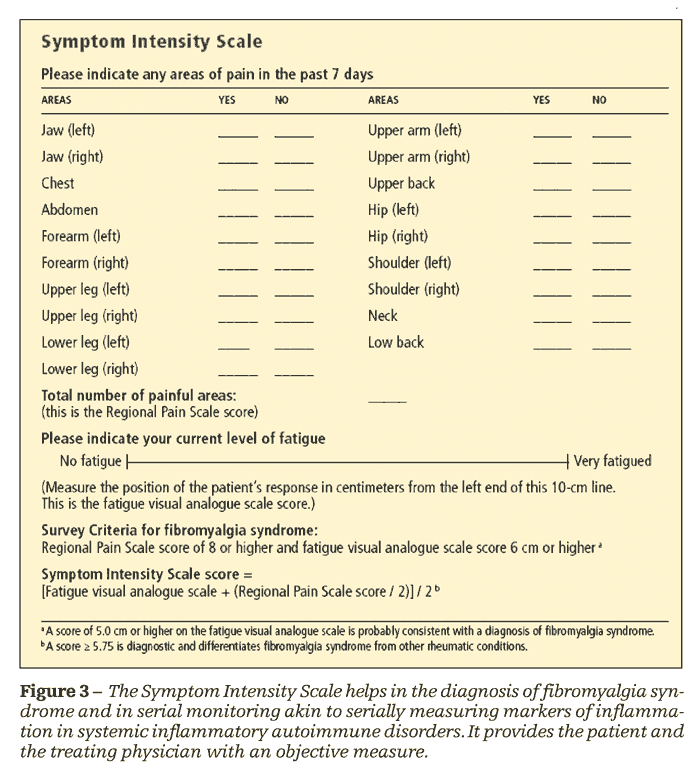
A subset of 19 nonarticular anatomical areas was found to distinguish FMS from the other diseases. These 19 areas constituted the Regional Pain Scale (RPS) part of the SIS; it was validated using appropriate statistical methods. A score of 8 or higher on the RPS, with 6 or higher on the FVAS, was observed to be precisely consistent with the diagnosis of FMS.
In 2006, Katz and associates9 observed a 75% concordance rate among the RPS measurements, the ACR criteria, and the clinical diagnosis of FMS (on the basis of a variety of symptoms). In another study, an RPS of 8 or higher had a sensitivity of 83.2% and a specificity of 87.6%; a score of 6 or higher on the FVAS also was useful for the diagnosis of FMS.
Wolfe and Rasker10 devised the formal SIS. This was calculated as the sum of the RPS divided by 2 and (referred to as the Symptom Intensity score, SIs) added to the FVAS; the total again divided by 2 [(RPS/2 + FVAS)/2]. The SIS refers to the scale; the SIs refers to the score. The calculated scores may range from 0 to 9.75; in this study of 25,417 patients, a cutoff score of 5.75 served to differentiate FMS from other rheumatologic diagnoses. The SIS also correlated well with the symptoms of FMS and with the Short Form–36, an objective measure of general health, mood, probability of having diabetes mellitus, requirement for hospitalization, rate of disability, and a slightly increased risk of premature death (relative risk, 1.12; 95% confidence interval, 1.10-1.14).
In assessing more than just pain and fatigue, the SIS leads to greater appreciation of the global impact of FMS on quality of life and serious morbidity and mortality. The impact of FMS on other rheumatologic diseases as well as on general health should be appreciated because it has profound implications about establishing the initial diagnosis and subsequent management of rheumatologic diseases. Use of the SIS allows the physician to better appreciate the biopsychosocial aspects in specific patients, to regard the whole patient. As movement toward new FMS criteria continues, tools like the SIS probably will minimize the diagnostic role of tender points.
Accompanying clinical conditions
Recognition of FMS may be aided by the presence of other conditions or symptoms. Pain often worsens with cold or humid weather, fatigue, a sedentary state, anxiety, or overactivity; it often is relieved by a warm shower, aerobic physical activity, and massage.
Conditions that may accompany FMS and be involved in its pathophysiology include postural orthostatic tachycardia syndrome, central sensitization (increased sensitivity to special senses stimuli), “interstitial cystitis,” carotodynia, irritable bowel syndrome, migraine headaches, a history of growing pains as a child, and sleep disorders. These associations lend plausibility to a common underlying central mechanism that a wide variety of specialists and primary care physicians who treat patients with FMS should appreciate. Although one of these symptoms may take center stage, thus leading to referral to a certain specialist, treating patients with accompanying conditions in a diagnostic vacuum may not be the best approach.
APPROACHES TO MANAGEMENT
FMS should not be a diagnosis of exclusion but instead a diagnosis of recognition; along with promoting better overall management, this approach may even prevent the use of potentially toxic therapy. For example, seeing persistent joint pain in the absence of swelling or objective synovitis and normal acute phase reactant levels in RA is common, in some cases because of comorbid FMS. Escalating immunosuppression in this situation is unnecessary, and doing so even may be hazardous. Rational treatment demands recognition and management of comorbid FMS.
The management of almost all medical conditions often is “individualized,” and FMS is no exception. Extra time spent at the initial encounter in attempting to identi-fy the whole patient profile may help future management. Extremely useful in initial assessment and serial follow-up are objective measures that use the SIS, Mood Disorders Questionnaire (MDQ), Patient Health Questionnaire (PHQ), Epworth Sleep Scale (ESS), Fibromyalgia Impact Questionnaire (FIQ), and other instruments. The management of FMS is complex, as is the underlying illness, and the recognition of certain phenotypes (predominant chronic pain, predominant chronic fatigue) allows for a better approach to therapy.
Patient education
Multimodal management of FMS starts with education, which is central to providing patients with insight into their illness and may facilitate adherence to therapeutic recommendations. Understanding their symptoms and possible underlying mechanisms may in itself be therapeutic, because patients with FMS often have seen multiple physicians and not received a unifying explanation of their illness. Setting realistic expectations about treatment options and goals at the onset of therapy is important. Although there is no generalized timeline for expected improvement of symptoms in all patients, it is necessary to see patients after 6 weeks to 3 months to subjectively and objectively reassess their response to initial therapy. As the components of FMS (pain, depression, and fatigue) change, so will FMS. Patients’ motivation and active participation in the therapeutic plan, resulting in shared decision making about their care, also are important.
The multimodal approach to therapy should be executed in parallel rather than in tandem, similar to the use of combination therapy rather than monotherapy from the time of diagnosis in patients with RA. Most patients are receptive to the hypothesized understanding of the mechanisms underlying their symptoms and willing to “do what it takes” to improve their quality of life. This helps break the vicious cycle of helplessness and fear that feeds depression and pain in patients with FMS. Formal educational materials may be provided for patients to read and reflect on after their visit.
Education should be emphasized as much as pharmacological and other nonpharmacological measures. It is helpful to acknowledge that although FMS is not in itself a “life-threatening” illness, its impact on quality of life is significant. Patients’ “individual FMS profiles” should be discussed because doing so helps set goals for therapy. Always encourage patients to become informed about their illness from reliable sources.
Involvement in local support groups provides an “outlet” for patients to discuss their symptoms and may help them find others with like symptoms. It also may enhance patient adherence to certain therapeutic recommendations.
Aerobic exercise
Regardless of the manifestations of FMS in a given patient, exercise is a keystone of treatment. A structured program of modest-intensity aerobic exercise helps maintain general fitness and physical conditioning. Most patients with FMS admit that they cannot participate in regular structured exercise because of the chronic pain and fatigue.
Patients who have had a reasonable level of premorbid exercise capacity feel even more symptomatic with pain and depression resulting from their inability to exercise because of their illness. Whether this increase stems from loss of a previously established set point of endogenous neurotransmitters or pain-regulating chemicals in a specific patient is not entirely clear. Most patients encounter the obstacle of experiencing increased pain with exercise initially and fatigue afterward.
Emphasize that exercise has a dose-dependent effect in FMS therapy and that reasonable benefit cannot be expected without achieving a certain intensity and duration of exercise. Encourage patients to escalate their intensity and increase their duration of exercise to recommended levels gradually because initial overenthusiasm may dampen the perceived benefit and lead to nonadherence later.
High-intensity nonaerobic exercise is not recommended for the management of FMS. Aerobic exercise helps improve physical conditioning and works as a valuable adjunct in the management of depression, anxiety, and stress.
The final goal of therapy is moderate-intensity aerobic exercise performed for at least 20 to 30 minutes about 5 times a week. Achieving this goal over time should be individualized because patients are more likely to be adherent with a shared plan of exercise.
For some patients, such exercise modalities as aquatic/pool therapy and riding a stationary bicycle, in addition to brisk walking, may be more realistic than others. Patients with trochanteric bursitis in particular may benefit from stationary bicycle exercises, and overweight patients may find that pool therapy helps them take weight off for better exercise. Biofeedback techniques are useful when combined with an exercise program.
Optimizing sleep
Another key issue in FMS management is sleep optimization. Most patients with FMS admit to sleep disturbances, such as difficulty in falling or maintaining sleep and feeling unrefreshed on wakening. Depression compounds the problem of poor sleep, making it necessary to address these closely related factors simultaneously.
Sleep disturbances in FMS may stem from primary sleep disorders or from obstructive sleep apnea. Patients in whom either problem is suspected should be convinced to undergo a formal sleep study to analyze sleep architecture and provide pertinent recommendations.
The goal of therapy is to optimize slow-wave (stage 3 or 4 nonrapid eye movement) sleep. Good sleep hygiene should be encouraged. Medications that may help sleep include low-dose tricyclic antidepressants (eg, amitriptyline and nortriptyline), trazodone, low-dose perphenazine, and doxepin taken at bedtime.
Other medications that may improve slow-wave sleep include pramipexole; some of the selec- tive serotonin reuptake inhibitors (SSRIs), such as fluoxetine; pregabalin; and 5-hydroxytryptophan. Benzodiazepine use should be discouraged because this agent actually reduces slow-wave sleep. Zopiclone and zolpidem have been shown to help sleep in FMS without compromising slow-wave sleep.
Managing depression
Because depression is a key aspect of the pathogenesis in many patients with FMS, it deserves investigation, recognition, and optimal management. Using the PHQ form facilitates recognition of depression and anxiety. The MDQ form helps clinicians recognize the tendency toward bipolar disorder. These scales provide objective measurement and serial assessment. Depression has an effect on sleep and the ability and motivation to exercise; therefore, if it is not addressed, it may derail other aspects of FMS management.
Insufficient serotonin and norepinephrine levels in the CNS have been identified as important mechanisms in the pathogenesis of FMS but not the only ones. Therefore, it is logical to attempt to restore the levels by pharmacological and nonpharmacological means.
The SSRIs and selective norepinephrine reuptake inhibitors (SNRIs) have been used to manage depression in patients with FMS. To manage depression in a specific patient, venlafaxine, paroxetine, escitalopram, and duloxetine may be used alone or in combination with the tricyclic antidepressants. If anxiety is a major component, buspirone may be a useful adjunct.
Low-dose quetiapine or aripiprazole alone or in combination with an SSRI or SNRI may be necessary in patients who have a bipolar disorder or tendency. These agents improve fatigue, facilitate control of depression and, in combination, protect these patients from experiencing manic episodes or cycle acceleration triggered by SSRI or SNRI treatment.11,12
Managing pain
Pain management in patients with FMS often is challenging. Patients should be counseled about pain mechanisms in FMS and that the pain that they experience may be associated with poor sleep, depression, and lack of exercise.
Opioid analgesics were investigated in a randomized controlled study and found to be ineffective.13 Their use should be strongly discouraged. If patients are already taking opioid analgesics, attempts should be made to wean them off these medications, perhaps with a formal pain management program.
Managing some of the other aspects of FMS may help decrease the pain to a “tolerable” level. Medications that may be used for pain management in FMS include low-dose tricyclic antidepressants, duloxetine, pregabalin, and pramipexole; combination therapy (tricyclic antidepressants with SSRIs or SNRIs) may be useful. In addition, aerobic exercise may increase CNS levels of endorphins, thus helping pain management.
A combination of measures
Use of a combination of nonpharmacological and pharmacological measures is important in the management of FMS. Patient participation should be enlisted as early as possible to ensure adherence to therapeutic recommendations. Filling out the above-mentioned questionnaires at serial visits helps provide objective feedback and encourages patients to start participating or further their participation in the management of their illness.
The use of pharmacological agents should be guided by the underlying predominant pathophysiology and symptoms in a given patient. Duloxetine, milnacipran, and pregabalin are some of the FDA-approved medications for FMS. However, these medications are by no means highly effective and should therefore be prescribed with a reasonable expectation of improvement.
In a study of 2228 patients treated with pregabalin, the median improvement in FIQ score was 14%.14 In another systematic review of the use of antidepressants in FMS, the reported median reduction in pain was 29% (range, 6% to 70%) and the median improvement in quality of life was 30% (range, 13% to 70%). In the same analysis, the median percentage of adverse effects was 72% (range, 14% to 100%).15 Informing patients to expect adverse effects is of course an important component of the conversation about treatment.
How we do it
•We do not consider FMS to be a diagnosis of exclusion.
•We recommend taking a detailed history and paying specific attention to various aspects of FMS and “the company it keeps.” A family history often reveals that other family members (eg, sisters, mother) have similar symptoms.
•We avoid liberal use of the term “fatigue”; on detailed questioning, it most often equates with depression.
•We attempt to identify triggers and acknowledge the impact of FMS on the patient’s quality of life.
•We try to identify the patient’s perception of FMS and which aspect of FMS is affecting him or her the most. Doing so helps in planning therapy goals.
•We ask the patient to fill out questionnaires at each visit (SIS, PHQ, MDQ, ESS) and discuss our interpretation of the scores.
•Using the history and patient questionnaires, we try to focus on managing the underlying “cause” rather than just the manifestations of FMS. This means identifying the factors that fuel FMS and attempting corrective measures.
•We try to explain FMS mechanisms to patients. One underlying mechanism of FMS is that for a per-unit nerve stimulus, patients experience an abnormally high and often distressful response. As an example, think of a cigarette lit in the basement of a building that sets off a smoke detector installed on the second floor. The underlying problem: the sensitivity of the smoke detector is set too low. The solution: raise the smoke detector’s threshold. One way to communicate this point to patients is to tell them, “I cannot cure you, but I can turn down the volume.”
•We emphasize the complexity of FMS and, accordingly, the complexity of treatment. Enlist patient understanding of the illness and participation in decision making about treatment measures. Set realistic goals.
•We refer the patient for sleep study, if indicated; physical therapy; or aquatic therapy. Most patients can motivate themselves for pool therapy. Refer the patient for sleep medicine if that is indicated by the results of the sleep questionnaire.
•We individualize pharmacological management on the basis of the patient’s profile (using the forms he or she filled out) and therapeutic goals.
•To achieve serial regular follow-up (office- or telephone-based), we sometimes provide patients with the questionnaires so that they can fill them out 6 to 8 weeks after the start of treatment and mail them back to us; then we can discuss the results with the patient and modulate treatment.
•We keep an open mind for other disorders. New symptoms are assessed on their own merit. We explain to patients that having FMS does not make them immune to other diseases and that they need to bring new symptoms to the attention of a physician.
•Basic focused laboratory work at the first visit helps us identify comorbid conditions that may be contributing to symptoms. We do not order extensive serological testing or imaging because FMS is a clinical diagnosis.
Is FMS a disease?
Patients and physicians often ask this question. Strictly speaking, FMS is an illness phenotype, a result of a complex interplay of central sensitization that owes its genesis and survival to depression, anxiety, poor sleep, genetic background, past experiences (eg, abuse, prematurity), and environmental factors.16 The pathogenic mechanisms are real/organic, and the resulting signs and symptoms equally so. Controversies exist in FMS as much as they do in other medical illnesses, and only time and further research will “separate the wheat from the chaff.” Until then, clinicians must work with their current understanding of this complex and challenging illness.
References:
References 1. Hench PK. Nonarticular rheumatism. Arthritis Rheum. 1976;19(suppl):1081-1088.
2. Smythe HA. “Fibrositis” as a disorder of pain modulation. Clin Rheum Dis. 1979;5:823-832.
3. Yunus M, Masi AT, Calabro JJ, et al. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched controls. Semin Arthritis Rheum. 1981;11:151-171.
4. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160-172.
5. MacFarlane GJ, Croft PR, Schollum J, Silman AJ. Widespread pain: is an improved classification possible? J Rheumatol. 1996;23:1628-1632.
6. White KP, Harth M, Speechley M, Ostbye T. Testing an instrument to screen for fibromyalgia syndrome in general population studies: the London Fibromyalgia Epidemiology Study Screening Questionnaire. J Rheumatol. 1999;26:880-884.
7. White KP, Speechley M, Harth M, Ãsbye T. The London Fibromyalgia Epidemiology Study (LFES): comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia syndrome (FMS) versus controls. J Rheumatol. 1999;26:1577-1585.
8. Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatol. 2003;30:369-378.
9. Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis: a comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum. 2006;54:169-176.
10. Wolfe F, Rasker JJ. The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. J Rheumatol. 2006;33:2291-2299.
11. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164:1348-1355.
12. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry. 2006;189:124-131.
13. Sörensen J, Bengtsson A, Bäckman E, et al. Pain analysis in patients with fibromyalgia: effects of intravenous morphine, lidocaine, and ketamine. Scand J Rheumatol. 1995;24:360-365.
14. Bennett RM, Bushmakin AG, Cappelleri JC, et al. Minimal clinically important difference in the fibromyalgia impact questionnaire. J Rheumatol. 2009;36:1304-1311.
15. Uçeyler N, Häuser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59:1279-1298.
16. Bradley LA. Pathophysiology of fibromyalgia. Am J Med. 2009;122(12 suppl):S22-S30.
17. Froriep R. Ein Beitrag zur Pathologie und Therapie des Rheumatismus. Weimar; 1843.
18. Gowers WR. A lecture on lumbago: its lessons and analogues. Br Med J. 1904;1:117-121.
19. Muller A. Untersuchungsbefund am rheumatisch erkrankten Muskel. Zeitschrift Klinische Medizin. 1912;74:34-73.
20. Lange F, Everbusch G. Die Bedeutung der Muskelharten für die allgemeine Praxis. Munich Med Wochenschr. 1921;68:418-420.
21. Reichart A. Reflexschmerzen auf grund von myoglosen. Dtsch Med Wochenschr. 1938;64:823-824.
22. Ruhman W. The earliest book on rheumatism. Br J Rheumatol. 1940;2:140-162.
23. Valentine M. Aetiology of fibrositis: a review. Ann Rheum Dis. 1947;6:241-250.
24. Travell J, Rinzler SH. The myofascial genesis of pain. Postgrad Med. 1952;11:425-434.




