Article
Do NSAIDs Impair Healing of Musculoskeletal Injuries?
NSAIDs are among the most frequently used and prescribed medications in the management of musculoskeletal pain and injury. Many physicians consider them to be the medication of choice. However, a number of studies have questioned the value of NSAIDs in the healing process of bone, muscle, tendon, and ligament injuries
ABSTRACT: Many physicians consider NSAIDs to be the medication of choice for managing musculoskeletal pain and injury. However, studies have questioned their value in the healing process of bone, muscle, tendon, and ligament injuries and their use carries the risk of potentially serious adverse effects. Animal and human studies have linked NSAID use to poor fracture healing. There appears to be little role for NSAIDs in tendinopathy outside of initial symptomatic pain relief. Animal studies provide conflicting evidence of efficacy in ligament injury, but human trials suggest that short courses may be of benefit in acute injury. Experimental animal models mostly demonstrate no effect on muscle healing or a reduction in muscle strength. Alternatives for analgesia in musculoskeletal injuries include acetaminophen, opiate-containing medication, and topical preparations. (J Musculoskel Med. 2011;28:207-212)
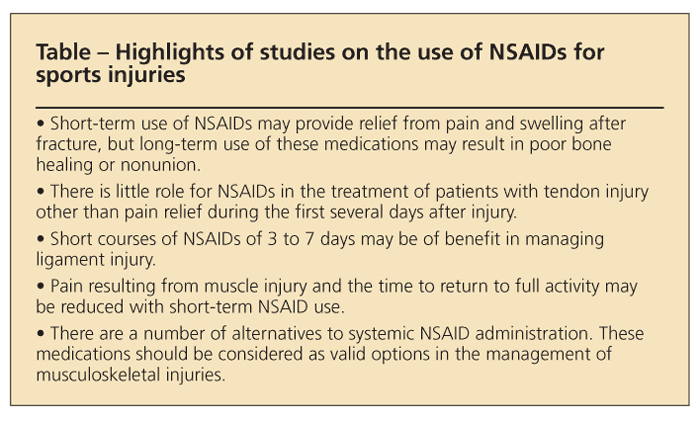
NSAIDs are among the most frequently used and prescribed medications in the management of musculoskeletal pain and injury. Many physicians consider them to be the medication of choice. However, a number of studies have questioned the value of NSAIDs in the healing process of bone, muscle, tendon, and ligament injuries (Table). In fact, there is evidence to suggest that long-term use of NSAIDs for managing fracture pain and inflammation carries the risk of impaired bone healing.
In addition, the use of over-the-counter or prescribed NSAIDs carries the risk of potentially serious adverse effects, particularly those that affect the GI tract and renal and cardiovascular (CV) systems. The introduction of cyclooxygenase (COX)-2 inhibitors, or coxibs, held the promise of providing the same therapeutic benefits as nonspecific NSAIDs-with fewer GI adverse effects. However, this class of medications has been shown to have potentially serious consequences for the CV system.1
In this article, we review how NSAIDs may negatively affect the healing process in musculoskeletal injuries. We also describe alternatives to systemic NSAIDs for analgesia in these injuries.
ACTION
The goal of NSAID use in the management of musculoskeletal injuries is reduction of pain and inflammation at the site of injury. NSAIDs act by inhibiting the action of COX, an enzyme responsible for the conversion of cell membrane arachidonic acid into prostaglandins, prostacyclins, and leukotrienes.1 These cellular mediators, distributed ubiquitously in many body tissues, help regulate diverse cellular processes, such as gastric mucus production, platelet aggregation, renal blood flow, neovascularization, vascular homeostasis, development of fever and inflammation, and modulation of pain receptors.2
Aspirin and other nonspecific NSAIDs act through the nonselective inhibition of both COX-1 and COX-2 enzymes to produce their biological effects. Coxibs act by selectively blocking the COX-2 isoform. Through the inhibition of the COX-2 enzyme, NSAIDs and coxibs curtail the formation of prostaglandins at the site of injury and thereby reduce pain, inflammation, elevation of body temperature, and recruitment of inflammatory cells.1
Common adverse effects of NSAIDs on the GI tract are the result of both direct irritation of the gastric mucosa and reduction of the protective actions of prostaglandins, which stimulate gastric mucus and bicarbonate production and reduce gastric acid secretion.3 NSAIDs also may cause renal impairment through alterations in renal blood flow and glomerular filtration, which can lead to renal failure.4
EFFECTFracture healing
Injuries to bone, particularly fractures, lead to a series of events that include hematoma formation and subsequent inflammatory response, development of granulation tissue and neovascularization, callus formation, bone deposition, and remodeling.5 Prostaglandins have been shown to play an important role in bone repair, which includes differentiation of osteoblast and osteoclast precursor cells, in addition to down-regulation of osteoclasts, leading to bone formation.6
In one study in rats, there appeared to be no difference in the histological appearance of healing bone over 21 days in the rats given ketoprofen or celecoxib compared with placebo.7 However, several other animal studies demonstrated that administration of etodolac,8 ibuprofen,9 or celecoxib10 has significant detrimental effects on bone healing as measured by histological or mechanical methods. These effects were most evident when NSAIDs were given for longer periods (3 to 12 weeks), but impairment of fracture healing was significant when celecoxib was given for just 1 week.10 The effect on bone healing was not evident with administration of acetaminophen.10 Other animal studies suggested that treatment with nonselective NSAIDs or coxibs has no effect on bone healing if it is given for 7 days11,12; longer treatment was associated with fracture nonunion.12
Human studies also have linked NSAID use to poor fracture healing. NSAIDs were found to be associated with nonunion or delayed healing of femoral shaft fractures in a study of 337 patients who took mainly ibuprofen or diclofenac for an average of 21 weeks.13 A large retrospective analysis of 10,000 patient records showed that patients who used NSAIDs during the first 3 months after a fracture experienced an almost 4-fold risk of fracture nonunion.14 However, a meta-analysis of high-quality observational studies conducted by Dodwell and associates15 concluded that the risk of fracture nonunion is not increased by treatment with NSAIDs. In another controlled trial, there appeared to be no difference in the time to recovery or subsequent bone density in postmenopausal women with a Colles fracture.16
NSAIDs have been used to reduce heterotopic bone formation in extraskeletal soft tissue after trauma, burns, or surgery. In a randomized controlled clinical trial that examined the effect of an NSAID or localized radiation to prevent heterotopic bone formation, administration of indomethacin was associated with nonunion of acetabular fractures after high-impact trauma in 26% of patients, compared with 7% who received radiation therapy.17 In addition, the use of local radiation was as effective as NSAID administration in preventing heterotopic ossification and was associated with fewer systemic adverse effects.18
When both the animal and human studies are taken into consideration, there is evidence to suggest that NSAIDs delay fracture healing when taken for more than 1 or 2 weeks. The effects of short-term use of NSAIDs remain to be investigated. The goal of early fracture treatment is short-term pain control until immobilization can be achieved. NSAIDs taken during the first 7 to 10 days after fracture may hasten the reduction of inflammation, allowing for earlier casting. Once this has been achieved, alternatives to NSAIDs for pain control should be considered.
Tendon injury
The treatment goal for tendinopathy, as for fractures, is decreased pain and return to normal function. The term “tendinopathy” has been associated with both chronic tendon degeneration (tendinosis) and acute injury (tendinitis). The majority of tendon disorders are deemed to be chronic, degenerative changes (tendinosis rather than tendinitis) and acute tendon injury resulting from overloading of tissue that already has undergone degenerative changes.19
Studies have demonstrated that prostaglandins and leukotrienes are produced during the acute phase of a tendon injury20 and may be involved in the subsequent degenerative changes over the long term. In the first few days after acute tendon injury, there is an initial inflammatory phase with angiogenesis, increased vascular permeability, and entry of inflammatory cells into the injury site.19 Prostaglandins are thought to be involved in these processes.2
However, in a study that examined the effect of celecoxib on rat ligament transaction, animals treated with this coxib for the initial 6 days after injury had weaker ligaments than those given placebo when tested 14 days after the initial injury.21 This result also was seen when treatment with coxibs for 14 days left injured ligaments significantly weaker than those treated with nonselective NSAIDs, acetaminophen, or placebo.22
In one study that examined the effects of NSAIDs on Achilles tendinopathy, patients treated with piroxicam fared no better in terms of pain, swelling, ankle stability, or muscle strength over a 4-week period than those who received placebo.23 In another study, however, NSAIDs resulted in improvement for patients with mild de Quervain disease when accompanied by splinting.24 Given that the pathophysiological process of tendinopathy is degenerative rather than primarily inflammatory as in de Quervain disease, there appears to be little role for NSAIDs outside of the initial symptomatic pain relief during the first few days after injury.
Ligament injury
The healing of damaged ligaments proceeds through 3 basic steps: (1) an initial inflammatory response that may be modified by NSAIDs, (2) a proliferative phase in which new collagen is produced, and (3) a subsequent remodeling phase. This entire process can encompass several months.6
Animal studies have produced conflicting evidence as to the potential benefits of NSAIDs on ligament injury. In rats who had undergone transaction of the medial collateral ligament (MCL), piroxicam given for 6 days resulted in a significant improvement in ligament healing.25 This effect was not seen with naproxen, rofecoxib, butorphanol, or acetaminophen.
In another study, animals with MCL injury were given ibuprofen or placebo for 14 or 28 days.26 There was no significant difference between the groups in the mechanical properties of the healed ligaments.
Few studies have examined the effects of NSAID treatment on ligament injury in humans. A randomized controlled trial of patients who had grade 1 or 2 ankle sprain and were treated with ibuprofen, celecoxib, or placebo demonstrated that treatment with celecoxib results in less pain and an earlier functional recovery.27 In a similar controlled trial, patients who received valdecoxib had less pain and resumed normal walking sooner than those given tramadol or placebo.28 However, patients who were given valdecoxib had significantly greater adverse effects than those in the tramadol group.
In an Australian study of army recruits who sustained ankle sprains, piroxicam was superior to placebo in pain reduction, time to return to training, and training endurance after injury.29 Other studies have demonstrated that both nonselective NSAIDs and coxibs are more effective than placebo for reducing pain in ankle ligament injuries and provide a more rapid return to activity.30,31 Animal studies provide conflicting evidence of the efficacy of NSAIDs in ligament injury, but human trials, which measured functional recovery, suggest that short courses of NSAIDs may be of benefit in acute ligament injury.
Muscle injury
Muscle and other soft tissue injuries-including muscle strains or tears, laceration, and other trauma to soft tissue-are common conditions in athletes for which they seek treatment. Muscle repair after injury involves 3 phases: (1) destruction, with hematoma formation and inflammatory reaction; (2) repair, in which myofibers regenerate and there is new blood vessel growth into the affected area; and (3) remodeling, with reorganization of the muscle architecture and subsequent recovery of function.32 Experimental animal models of muscle injury used to examine how NSAIDs may affect healing mostly demonstrated no effect on muscle healing or a reduction in muscle strength and altered cytoarchitecture after injury.33-37
Most studies in humans examined the effect of short courses of NSAIDs in acute muscle injury. A study that examined the duration of muscle soreness after eccentric muscle exercise reported that ibuprofen received postactivity had little effect on muscle soreness 24 and 28 hours after exercise.38 Patients who were given diclofenac before a strenuous exercise program had less histological muscle damage than patients who received placebo.39
A small study examined the effect of 10 days of treatment with naproxen on quadriceps muscle soreness after strenuous eccentric exercise.40 The naproxen group had a significantly shorter duration of muscle soreness and less swelling than patients given placebo. However, in patients with acute hamstring muscle injury who were undergoing physiotherapy, meclofenamate or diclofenac administration had little effect on pain assessment or muscle performance.41 Two small studies in older patients showed a positive effect of NSAIDs on muscle injury after exercise.42,43
In all, the short-term use of NSAIDs in muscle injury reduces pain and the time to return to full activity. However, long-term use of these agents is not likely to convey additional benefits and may result in a higher incidence of GI and CV adverse effects.
ALTERNATIVES TO
SYSTEMIC NSAIDs
Alternatives to NSAIDs for analgesia in musculoskeletal injuries include acetaminophen, opiate-containing medication, and topical preparations. A review of several small studies concluded that acetaminophen is as effective as NSAIDs for pain reduction after musculoskeletal injury.44
Warden45 noted in a review that there is a lack of scientific evidence to support the use of prophylactic NSAIDs to prevent inflammation and pain that may accompany training and activity. Also, the potential adverse effects on the GI and CV systems outweigh any perceived benefits from using NSAIDs in this way.
Boger and Jones46 proposed an interesting approach to escalating analgesic treatment in acute musculoskeletal injury. In that review, use of acetaminophen was advocated as the first-line analgesic. If adequate pain control was not achieved, then codeine would be added to the acetaminophen. The next step up in management would be to replace codeine with NSAIDs.47 This was written with the view that codeine is available over-the-counter, as it is in many countries, including the United Kingdom; however, prescribing practices in the United States may not allow for ready adoption of this schema.
A systematic review of randomized controlled trials reported that topical NSAIDs and coxibs significantly reduced pain and resulted in low incidences of systemic and local adverse effects.48 Subsequent studies have supported the notion that topical NSAIDs are effective for pain reduction after acute soft tissue injuries.44
CONCLUSION
Although evidence suggests that long-term use of NSAIDs for managing fracture pain and inflammation carries the risk of impaired bone healing, the use of NSAIDs for shorter periods probably would have little impact on the overall healing process. Therefore, these medications may still be of benefit in patients who cannot take other types of analgesics.
In tendinopathies, where inflammation plays a lesser role, NSAIDs probably have little influence on healing and may be of some benefit as a limited short course for analgesia. With sprains, strains, or tears of ligaments, NSAIDs may be of more use by limiting the pain and swelling of these injuries, increasing patients’ chances of regaining function and returning to activity sooner. Studies have shown some value in this therapy when used for short periods (3 to 7 days). Evidence also supports the short-term use of NSAIDs for the management of muscular injury, providing some pain relief and allowing for earlier resumption of normal activity.
NSAIDs carry potentially serious adverse effects that could affect the GI, CV, and renal systems, leading to significant morbidity. Careful consideration should be given to these effects befor prescribing or recommending NSAIDs for patients who have bone and soft tissue injuries.
References:
References
1. Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8:390-396.
2. Mehallo CJ, Drezner JA, Bytomski JR. Practical management: nonsteroidal antiinflammatory drug (NSAID) use in athletic injuries. Clin J Sport Med. 2006;16:170-174.
3. Lazzaroni M, Porro GB. Management of NSAID-induced gastrointestinal toxicity: focus on proton pump inhibitors. Drugs. 2009;69:51-69.
4. Baker J, Cotter JD, Gerrard DF, et al. Effects of indomethacin and celecoxib on renal function in athletes. Med Sci Sports Exerc. 2005;37:712-717.
5. Radi ZA, Khan NK. Effects of cyclooxygenase inhibition on bone, tendon, and ligament healing. Inflamm Res. 2005;54:358-366.
6. Kawaguchi H, Pilbeam CC, Harrison JR, Raisz LG. The role of prostaglandins in the regulation of bone metabolism. Clin Orthop Relat Res. 1995;313:36-46.
7. Matsumoto MA, De Oliveira A, Ribeiro Junior PD, et al. Short-term administration of non-selective and selective COX-2 NSAIDs do not interfere with bone repair in rats. J Mol Histol. 2008;39:381-387.
8. Endo K, Sairyo K, Komatsubara S, et al. Cyclooxygenase-2 inhibitor delays fracture healing in rats. Acta Orthop. 2005;76:470-474.
9. O’Connor JP, Capo JT, Tan V, et al. A comparison of the effects of ibuprofen and rofecoxib on rabbit fibula osteotomy healing. Acta Orthop. 2009;80:597-605.
10. Bergenstock M, Min W, Simon AM, et al. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J Orthop Trauma. 2005;19:717-723.
11. Utvåg SE, Fuskevåg OM, Shegarfi H, Reikerås O. Short-term treatment with COX-2 inhibitors does not impair fracture healing. J Invest Surg. 2010;23:257-261.
12. Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, et al. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing: reversibility of effects after short-term treatment. J Bone Joint Surg. 2007;89A:114-125
13. Giannoudis PV, MacDonald DA, Matthews SJ, et al. Nonunion of femoral diaphysis: the influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg. 2000;82B:655-658.
14. Bhattacharyya T, Levin R, Vrahas MS, Solomon DH. Nonsteroidal antiinflammatory drugs and nonunion of humeral shaft fracture. Arthritis Rheum. 2005;53:364-367.
15. Dodwell ER, Latorre JG, Parisini E, et al. NSAID exposure and risk of nonunion: a meta-analysis of case-control and cohort studies. Calcif Tissue Int. 2010;87:193-202.
16. Adolphson P, Abbaszadegan H, Jonsson U, et al. No effects of piroxicam on osteopenia and recovery after Colles’ fracture: a randomized, double-blind, placebo-controlled, prospective trial. Arch Orthop Trauma Surg. 1993;112:127-130.
17. Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg. 2003;85B:700-705.
18. Kienapfel H, Koller M, Wüst A, et al. Prevention of heterotopic bone formation after total hip arthroplasty: a prospective randomized study comparing postoperative radiation therapy with indomethacin medication. Arch Orthop Trauma Surg. 1999;119:296-302.
19. Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg. 2005;87A:187-202.
20. Riley G. Tedinopathy-from basic science to treatment. Nat Clin Pract Rheumatol. 2008;4:82-89.
21. Elder CL, Dahners LE, Weinhold PS. A cyclooxygenase-2 inhibitor impairs ligament healing in the rat. Am J Sports Med. 2001;29:801-805.
22. Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35:1326-1333.
23. Aström M, Westlin N. No effect of piroxicam on achilles tendinopathy: a randomized study of 70 patients. Acta Orthop Scand. 1992;63:631-634.
24. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
25. Dahners LE, Gilbert JA, Lester GE, et al. The effect of nonsteroidal antiinflammatory drug on the healing of ligaments. Am J Sports Med. 1988;16:641-646.
26. Moorman CT 3rd, Kukreti U, Fenton DC, Belkoff SM. The early effect of ibuprofen on the mechanical properties of healing medial collateral ligament. Am J Sports Med. 1999;27:738-741.
27. Ekman EF, Fiechtner JJ, Levy S, Fort JG. Efficacy of celecoxib versus ibuprofen in the treatment of acute pain: a multicenter, double-blind, randomized controlled trial in acute ankle sprain. Am J Orthop (Belle Mead NJ). 2002;31:445-451.
28. Ekman EF, Ruoff G, Kuehl K, et al. The COX-2 specific inhibitor Valdecoxib versus tramadol in acute ankle sprain: a multicenter randomized, controlled trial. Am J Sports Med. 2006;34:945-955.
29. Slatyer MA, Hensley MJ, Lopert R. A randomized controlled trial of piroxicam in the management of acute ankle sprain in Australian Regular Army recruits. The Kapooka Ankle Sprain Study. Am J Sports Med. 1997;25:544-553.
30. Morán M. Double-blind comparison of diclofenac potassium, ibuprofen and placebo in the treatment of ankle sprains. J Int Med Res. 1991;19:121-130.
31. Petrella R, Ekman EF, Schuller R, Fort JG. Efficacy of celecoxib, a COX-2-specific inhibitor, and naproxen in the management of acute ankle sprain: results of a double-blind, randomized controlled trial. Clin J Sport Med. 2004;14:225-231.
32. Järvinen TA, Järvinen TL, Kääriäinen M, et al. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745-764.
33. Järvinen M, Lehto M, Sorvari T. Effect of some anti-inflammatory agents on the healing of ruptured muscle: an experimental study in rats. J Sport Traumatol Rel Res. 1992;14:19-28.
34. Rahusen FT, Weinhold PS, Almekinders LC. Nonsteroidal anti-inflammatory drugs and acetaminophen in the treatment of an acute muscle injury. Am J Sports Med. 2004;32:1856-1859.
35. Mishra DK, Fridén J, Schmitz MC, Lieber RL. Anti-inflammatory medication after muscle injury: a treatment resulting in short-term improvement but subsequent loss of muscle function. J Bone Joint Surg. 1995;77A:1510-1519.
36. Shen W, Prisk V, Li Y, et al. Inhibited skeletal muscle healing in cyclooxygenase-2 gene-deficient mice: the role of PGE2 and PGF2alpha. J Appl Physiol. 2006;101:1215-1221.
37. Shen W, Li Y, Tang Y, et al. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am J Pathol. 2005;167:1105-1117.
38. Rahnama N, Rahmani-Nia F, Ebrahim K. The isolated and combined effects of selected physical activity and ibuprofen on delayed-onset muscle soreness.J Sports Sci. 2005;23:843-850.
39. O’Grady M, Hackney AC, Schneider K, et al. Diclofenac sodium (Voltaren) reduced exercise-induced injury in human skeletal muscle. Med Sci Sports Exerc. 2000;32:1191-1196.
40. Dudley GA, Czerkawski J, Meinrod A, et al. Efficacy of naproxen sodium for exercise-induced dysfunction muscle injury and soreness. Clin J Sport Med. 1997;7:3-10.
41. Reynolds JF, Noakes TD, Schwellnus MP, et al. Non-steroidal anti-inflammatory drugs fail to enhance healing of acute hamstring injuries treated with physiotherapy. S Afr Med J. 1995;85:517-522.
42. Lanier AB, Simpson KJ, Gregory C, et al. Exercise-induced muscle injury and influence of NSAID therapy on kinematics of downhill walking in older adults. JEPonline. 2009;12:11-21.
43. Baldwin AC, Stevenson SW, Dudley GA. Nonsteroidal anti-inflammatory therapy after eccentric exercise in healthy older individuals. J Gerontol A Biol Sci Med Sci. 2001;56:M510-M513.
44. Feucht CL, Patel DR. Analgesics and anti-inflammatory medications in sports: use and abuse. Pediatr Clin North Am. 2010;57:751-774.
45. Warden SJ. Prophylactic use of NSAIDs by athletes; a risk/benefit assessment. Phys Sportsmed. 2010;38:132-138.
46. Boger EJ, Jones AK. Paracetamol use in musculoskeletal pain: an audit of use and patient perceptions of paracetamol as an effective analgesic. Musculoskeletal Care. 2005;3:224-232.
47. Braund R, Abbott JH. Analgesic choice when treating musculoskeletal sprains and strains. N Z J Physiother. 2007;35:54-60.
48. Moore RA, Tramèr MR, Carroll D, et al. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs [published correction appears in BMJ. 1998;316:1059]. BMJ. 1998;316:333-338.




