Article
Early intervention in rheumatoid arthritis pays off
<em>The outlook for patients with newly diagnosed rheumatoid arthritis (RA) has improved greatly, but many questions remain, including when to initiate therapy and what drugs to use. Recently issued recommendations for managing early RA include referring patients who present with arthritis of more than 1 joint to a rheumatologist. Combination's of drugs have been shown to be more effective than monotherapy. The tumor necrosis factor α inhibitors have revolutionized treatment, and other biologic agents are available for those who have an inadequate response. Making the diagnosis early often presents a challenge. Recently, a prediction rule was published for patients with undifferentiated arthritis of recent onset. The imaging focus for early arthritis has shifted from radiographs to ultrasonography and MRI. (J Musculoskel Med. 2008;25:70-76) </em>
ABSTRACT: The outlook for patients with newly diagnosed rheumatoid arthritis (RA) has improved greatly, but many questions remain, including when to initiate therapy and what drugs to use. Recently issued recommendations for managing early RA include referring patients who present with arthritis of more than 1 joint to a rheumatologist. Combination's of drugs have been shown to be more effective than monotherapy. The tumor necrosis factor α inhibitors have revolutionized treatment, and other biologic agents are available for those who have an inadequate response. Making the diagnosis early often presents a challenge. Recently, a prediction rule was published for patients with undifferentiated arthritis of recent onset. The imaging focus for early arthritis has shifted from radiographs to ultrasonography and MRI. (J Musculoskel Med. 2008;25:70-76)
In the past, the diagnosis of rheumatoid arthritis (RA) often foretold a future of joint damage resulting in a patient’s loss of function and inability to participate in the workforce,1 decreased life expectancy, and increased health care costs.2 However, the outlook for patients with newly diagnosed RA has improved greatly over the past 20 years.3 The advent of earlier treatment, the use of disease-modifying antirheumatic drugs (DMARDs) in combination, and the use of biologic response modifiers have been shown to slow the progression of radiographic joint damage and maintain or improve physical function in patients who present with RA.4
Current patients with RA are less likely than patients in 1985 to have significant work disability, decreased physical function, and premature mortality.3 However, many questions remain about when to initiate therapy, what combination of drugs to use, how to monitor disease activity, and when to adjust therapy. In this article, I attempt to provide some useful answers.
Clarifying early arthritis
What is early arthritis? How is it different from RA that fulfills the American College of Rheumatology (ACR) criteria? How should it be managed and measured? In a recent report, a select European League Against Rheumatism (EULAR) committee took on the daunting task of answering these questions. From their hard work arose a welcomed clarity about the complicated and evolving area of early arthritis.
The study used a standard EULAR approach of asking 14 rheumatology experts from 10 European countries to generate matters of interest about the management of early arthritis. From this process arose 12 management recommendations.
This clinical road map to early arthritis, supported by proper legends and mileposts, was the first of its kind. Early management of RA has become a mantra, but how to address the earliest stirrings of joint inflammation that can resolve or evolve into RA has heretofore involved extrapolating from lessons learned with RA.1,2
A fitting first recommendation was that patients presenting with arthritis of more than 1 joint should be referred to and seen by a rheumatologist, ideally within 6 weeks after the onset of symptoms. Recommendations also included the following:
• The clinical examination is the method of choice for detecting arthritis (ultrasonography, MRI, or power Doppler may be used in difficult cases).
• Some screening laboratory tests (eg, antinuclear antibodies) are needed for exclusion of other disorders, such as systemic lupus erythematosus.
• Factors that predict persistent or erosive disease (eg, anti-cyclic citrullinated peptide [anti-CCP], rheumatoid factor [RF]) should be measured, and patients at risk for persistent or severe RA need to be treated immediately and with NSAIDs, short courses of corticosteroids or intra-articular corticosteroids, and the anchor drug methotrexate (MTX), aiming for remission.
In spite of cardiovascular, GI, and other concerns about NSAIDs and cyclooxygenase 2 selective inhibitors, most physicians in my division of 44 rheumatologists still include an NSAID in the regimen and add a proton pump inhibitor or, if needed, low-dose aspirin. As always, the physician in partnership with an informed patient must make cost-benefit decisions about all medications, including NSAIDs.
• Monitoring of disease activity (using tender/swollen joint counts, patient and physician global assessments, C-reactive protein level [CRP], erythrocyte sedimentation rate [ESR], the Health Assessment Questionnaire [HAQ], or the Disease Activity Score [DAS]) is mandatory every 1 to 3 months; in addition, joint damage assessment using plain x-ray films every 6 to 12 months is needed.
• Treatment adjuncts (eg, dynamic exercises) may be applied along with pharmacological measures.
Rapid, early progression
Early, intensive RA treatment was first recommended in the 1970s when gold was shown to be an effective disease-modifying agent.5,6 It is now well established that within 2 years of disease onset, up to 93% of persons with RA manifest radiographic evidence of significant, irreversible joint damage.7-10 The rate of RA progression is significantly more rapid in the first year than in the second and third years,8 and erosions may be detected by MRI within 4 months of disease onset (Figure 1).7 Therefore, early intervention is critical in slowing disease progression and improving physical function.11
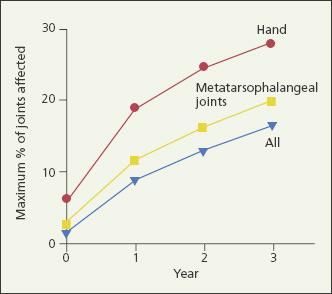
Figure 1 – The percentage of joints that are affected by rheumatoid arthritis (RA) in the first 3 years after disease onset is shown in this graph. The rate of RA progression is significantly more rapid in the first year than in the second and third years. Early intervention is critical in slowing disease progression.
Initiating DMARD therapy
The traditional DMARDs-MTX, sulfasalazine (SSZ), leflunomide, corticosteroids, cyclosporine, and injectable gold salts-have been shown to slow radiographic progression in patients with RA.12 In addition, combinations of DMARDs have been shown to be more effective in suppressing disease activity than monotherapy.11,13-15 Calgneri and associates13 found that combination therapy with MTX, SSZ, and hydroxychloroquine (HCQ) stopped the radiographic progression of active RA in nearly 70% of treated patients, compared with nearly 25% of patients treated with MTX, SSZ, or HCQ monotherapy (Figure 2). Together, these findings would suggest that DMARDs are best initiated early and in combination.
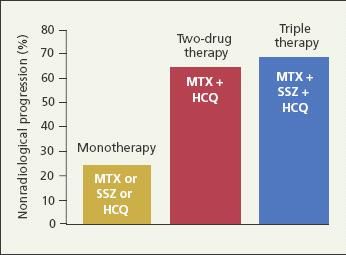
Figure 2 –
Combinations of disease-modifying antirheumatic drugs have been shown to be more effective in suppressing disease activity in rheumatoid arthritis than monotherapy. Shown in this bar graph is the efficacy of monotherapy versus combination therapy in slowing radiographic progression in the first 24 months of treatment. (MTX, methotrexate; SSZ, sulfasalazine; HCQ, hydroxychloroquine.) (Adapted from Calgneri M et al.
Clin Exp Rheumatol.
1999.13)
This was borne out in the Finnish Rheumatoid Arthritis Combination (FIN-RACo) therapy trial.15 FIN-RACo was a multicenter, randomized controlled trial that compared combination DMARD treatment (MTX, SSZ, and HCQ plus prednisone) with single DMARD therapy (SSZ with and without prednisone or MTX with and without prednisone) in the management of early RA. The primary outcome was remission, defined as the absence of tender or swollen joints and pain, less than 15 minutes of morning stiffness, and a normal ESR. Significantly more patients receiving combination versus single DMARD treatment achieved remission, and they did so without a greater frequency of adverse events.15
The Combinatietherapie Bij Reumatoide Artritis (COBRA) trial also investigated the efficacy of combination DMARD therapy in early RA.11 However, this trial took the early treatment model one step further and evaluated whether remission could be induced and medication withdrawal tolerated if patients received early, aggressive therapy.
COBRA was a multicenter, randomized, double-blind trial that compared combination MTX, SSZ, and prednisone with SSZ alone in patients with early RA (median duration, 4 months). In the combination group, prednisone and MTX were tapered and stopped at 28 and 40 weeks, respectively. Significantly more patients in the combination group (49%) than in the SSZ group (27%) achieved an ACR 50 response (P = .007). The number who achieved remission by a mean pooled index was not significantly different between groups, and the clinical difference between groups after the prednisone was stopped was not significant.11
Notably, however, 4- to 5-year follow-up showed that those who received the COBRA intensive, short-term combination treatment had a slower rate of radiographic progression compared with the SSZ group after controlling for consecutive DMARD therapy and differences in disease activity.16 This finding supports the “window of opportunity” hypothesis that early, aggressive therapy may have a long-term effect on radiographic progression and that patients with early disease have a more favorable response to treatment.17
The Tight Control for Rheumatoid Arthritis (TICORA) study demonstrated that with intensive symptom monitoring, RA remission could be induced with traditional DMARDs at no greater cost than that of routine care.18 The TICORA study compared intensive monitoring (monthly visits to a rheumatologist, intra-articular and intramuscular corticosteroid injections, and rapid transition from monotherapy with SSZ to triple therapy with SSZ, HCQ, and MTX) with routine care (visits every 3 months without formal measure of disease activity and serial monotherapy with DMARDs, followed by combination therapy at the discretion of the practitioner). At 18 months, 65% of patients in the intensive management group had achieved remission by EULAR criteria, compared with 16% in the routine care group (P < .0001).
Tumor necrosis factor α inhibitors
Although combination therapy with traditional DMARDs has greatly improved disease control and induced remission in some patients, the development of tumor necrosis factor α (TNF-α) inhibitors in the late 1990s revolutionized the treatment of patients with RA.19 Large randomized controlled trials of 3 TNF-α inhibitors (infliximab, etanercept, and adalimumab) showed that combination treatment with a TNF-α inhibitor and MTX is superior to monotherapy with MTX for improving clinical symptoms and slowing radiographic progression in patients with RA of less than 3 years’ duration.20-22 The Trial of Etanercept and Methotrexate With Radiographic Patient Outcomes and PREMIER trial also showed that combination therapy with MTX and etanercept or adalimumab is superior to therapy with either TNF-α inhibitor alone.21,22 Although combination therapy was shown to be superior, monotherapy with MTX, etanercept, or adalimumab was not ineffective, leaving open the question of which treatment strategy to use in patients who present with RA.
Can a patient who is not successful with one TNF-α inhibitor be switched to another? Despite impressive clinical and radiological outcomes achieved with the use of these medications, one third of patients show a lack of response because of a lack of drug efficacy or with the development of adverse events. Although these drugs all achieve their benefits through TNF-α blockade, they vary in their site of immunological action and molecular structure.
In a recent study that involved a prospective cohort of 6739 patients treated with TNF-α inhibitors over a 15-month period, 841 and 1023 of them stopped taking the first drug because of inefficacy and toxicity, respectively. Of these patients, 503 and 353, respectively, were switched to a second TNF-α inhibitor. Overall, 73% of patients who switched were still taking the new therapy by the end of the 6-month follow-up.
First drug discontinuation because of lack of efficacy was associated with an increased risk of second drug discontinuation for the same reason. Similarly, first drug discontinuation because of toxicity was associated with an increased rate of second drug discontinuation because of toxicity, not inefficacy.
Further studies will be needed to determine whether there is a role for yet a third TNF-α inhibitor. However, once a patient with RA has not responded to 2 TNF-α inhibitors, most rheumatologists believe that that patient’s RA is not “driven by TNF” and that the use of another B- or T-cell–specific agent is appropriate.23
The suggestion of widespread implementation of TNF-α inhibitor therapy for early RA has raised cost and safety concerns. A TNF-α inhibitor may cost between $12,000 and $30,000 per year, limiting access to the drug.24 Given the role of TNF-α in the immune system, an increased incidence of infection and malignancy associated with this category of therapy is not surprising. Postmarketing surveillance has shown this therapy to be associated with infusion or injection site reactions, tuberculosis (TB), lymphoma autoimmune disorders, cardiac insufficiency, demyelinating disease, and interstitial lung disease.25
Currently, all patients are screened for TB before the start of TNF-α inhibitor treatment. Although an elevated risk of lymphoma remains a concern for all patients with active RA,26 a recent Brigham and Women’s Hospital study concluded that patients whose RA was managed with a TNF-α inhibitor were at no greater risk for lymphoma than those whose disease was managed with MTX.27
The Behandel Strategien (BeSt) study was designed to determine which aggressive treatment strategy is most effective in patients who present with RA.28 In this multicenter, randomized, unblinded clinical trial, patients were assigned to 1 of 4 treatment groups: (1) sequential monotherapy starting with MTX; (2) step-up combination therapy: MTX with additional SSZ, HCQ, and prednisone, as needed; (3) initial combination therapy (MTX with SSZ) with tapered high-dose prednisone; or (4) initial combination therapy with MTX and infliximab. Study physicians changed and adjusted medications based on patients’ DAS in 44 joints.
The 1-year data showed that initial treatment with prednisone or infliximab results in earlier functional improvement and less radiographic damage without increased toxicity.28 The 2-year data showed that with close monitoring and guidelines for changing treatment, patients achieve similar disease control regardless of initial treatment assignment.4 Although these results might cause some to advocate initial monotherapy with tight disease control, the authors noted that disease activity within the first year can have a significant economic impact, because those with active disease often are unable to work.4,29 They also noted that patients in the sequential monotherapy group sustained more irreversible radiographic damage within the first year.4
The 4-year follow-up of the BeSt study demonstrated that initial treatment with MTX and infliximab in early-onset RA is more effective than reserving MTX and infliximab for patients who did not have success with previous DMARDs, resulting in more reduction of DAS and HAQ scores, more patients in clinical remission, and more successful discontinuation of infliximab in the patients treated with initial infliximab. This observation supports the window of opportunity for antirheumatic therapy theory.
Other biologic therapies
The current goal for management of RA is remission. Although many patients with early RA respond to traditional DMARDs, TNF-α inhibitors, or both, other biologic agents are available for those who have an inadequate response to these agents. All these agents may be used in combination with MTX but not with TNF-α inhibitors because of the increased risk of infection.
Anakinra, which is administered as a daily subcutaneous injection, inhibits interleukin-1. This agent used alone or in combination with MTX has been shown to be more effective than placebo in the management of RA for patients who have not succeeded with traditional DMARDs or TNF-α inhibitors.1
Rituximab is a chimeric antibody against the CD20 molecule on B lymphocytes; its effect is related to B-cell depletion. Early (24-week) results of the Randomized Evaluation of Long-Term Efficacy of Rituximab trial (a randomized, double-blind, placebo-controlled phase 3 trial of rituximab) suggested that patients with long-standing, active RA who have had inadequate response to 1 or more TNF-α inhibitors have significant clinical improvement with rituximab compared with placebo.2
Abatacept (human cytotoxic T-lymphocyte–associated antigen 4 linked to the modified Fc portion of human IgG1) inhibits T-cell activation. A randomized, double-blind phase 3 trial of abatacept versus placebo plus traditional DMARDs showed significant clinical and functional improvement in patients who had inadequate response to at least 3 months of TNF-α inhibitors.3
Predicting disease severity in early RA
Although early, aggressive treatment is indicated in RA, making the diagnosis at an early stage often presents a challenge. Many patients with RA of recent onset do not fulfill any classification criteria. Cohort studies have shown that RA will develop in 6% to 55% of patients who present with undifferentiated inflammatory arthritis, but 40% to 50% have spontaneous remission of symptoms.30-32 Given that early intervention can prevent permanent damage, identifying the patients at high risk for future erosive disease is critical. A reliable method of risk stratification would permit treatment to be directed to those whose RA is most likely to progress and reduce the frequency of drug-related adverse effects in those whose RA is most likely to abate.
Until recently, no effective models for predicting disease severity had been proposed. In 2007, however, van der Helm-van Mil and colleagues33 published a prediction rule for patients with undifferentiated arthritis of recent onset. This rule used 9 clinical variables: age, sex, localization of arthritic symptoms, morning stiffness, tender joint count, swollen joint count, CRP level, RF positivity, and the presence of anti-CCP.
The prediction score ranged from 0 to 14; an upper cutoff value of 8 had a positive predictive value of 84%, and a lower cutoff level of 6 had a negative predictive value of 91%.33 In the validation cohort, 100% of patients with a score of 8 or greater progressed to RA; RA did not develop during 1 year of follow-up in 94% of patients with a score of 6 or lower.33 Although individualized treatment must be based on physician discretion, this prediction rule offers a good starting point for making treatment decisions about patients with undifferentiated arthritis.
Monitoring response to therapy
In addition to clinical predictors, rheumatologists have long sought a sensitive and reliable imaging method to assess RA progression and to monitor response to treatment. Traditionally, standard radiographs of the hands and feet have been used to detect erosions. However, up to 70% of patients who present with early inflammatory arthritis have normal radiographic results at the initial visit because conventional radiographs lack the sensitivity and specificity needed to detect bone erosions and joint space loss early in disease.8,34 In recent years, the imaging focus for early arthritis has shifted from radiographs to ultrasonography and MRI, both of which are more sensitive for detecting early erosive disease and offer more information about the soft tissues and synovium.35,36
Ultrasonography is an attractive imaging modality in early inflammatory arthritis because it is somewhat portable, does not use ionizing radiation, and is less expensive than MRI.37 This modality may be used to quantify synovial thickening, joint effusions, and bone erosions in patients with RA.35 Power Doppler ultrasonography (PDU), a more powerful technique, enables clinicians to assess the vascularity of the synovial membrane and identify erosions. Studies that used both MRI and histological findings as a standard have demonstrated PDU to be both sensitive and specific for this purpose.38-40 Disadvantages of ultrasonography include its operator dependency, variability of the machines and imaging processing parameters, and a lack of standardized protocols for interpretation.
MRI is starting to play a much larger role in the early diagnosis and management of RA because it can depict the synovium, tendons, ligaments, and cartilage, in addition to bone. The synovium is the primary site at which inflammatory mediators effect change in early disease. Inflammatory cellular infiltrate and vascular congestion cause an increase in synovial volume. MRI can image the synovium directly and detect subchondral marrow changes and erosions at a much earlier stage than conventional radiographs.41 Periarticular areas of bone marrow edema have been identified as sites of early reaction to synovitis and precursors to erosive disease.7,42
Changes that resemble small bone erosions or mild synovitis may be seen on MRI performed on healthy persons. This possibility has raised concerns about the significance of erosions seen on MRI.43 However, the bone erosions and bone marrow edema visible on contrast-enhanced MRI are not seen in healthy subjects. Therefore, such findings have great potential to focus treatment in early inflammatory arthritis.43
Like ultrasonography, MRI has disadvantages, including cost, patient discomfort (noise, claustrophobia, and awkward positioning), limited access (to machines or to specialized radiologists in some countries), and the need to use contrast agents.37 The Outcome Measures in Rheumatoid Arthritis Clinical Trial group has worked to develop standardized sequences and interpretation of MRI images.44 Low-field, office-based MRI machines may address some of the problems of expense and patient discomfort.37
Improving outcomes
With growing awareness of the need for early intervention in RA and continuing advances in treatment, outcomes continue to improve, although early identification of patients at high risk for erosive disease remains a challenge. As more is learned more about risk factors for disease severity and as the role and utility of ultrasonography and MRI are better defined, remission will become a reality for more patients who have early inflammatory arthritis.
References:
References
- 1. Pincus T, Callahan LF, Sale WG, et al. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864-872.
- 2. Wong JB, Ramey DR, Singh G. Long-term morbidity, mortality, and economics of rheumatoid arthritis. Arthritis Rheum. 2001;44:2746-2749.
- 3. Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum. 2005;52:1009-1019.
- 4. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146:406-415.
- 5. Luukkainen R, Kajander A, Isomaki H. Effect of gold on progression of erosions in rheumatoid arthritis: better results with early treatment. Scand J Rheumatol. 1977;6:189-192.
- 6. Lehman AJ, Esdaile JM, Klinkhoff AV, et al; METGO Study Group. A 48-week, randomized, double-blind, double-observer, placebo-controlled multicenter trial of combination methotrexate and intramuscular gold therapy in rheumatoid arthritis: results of the METGO study. Arthritis Rheum. 2005;52:1360-1370.
- 7. McQueen FM, Stewart N, Crabbe J, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann Rheum Dis. 1998;57:350-356.
- 8. van der Heijde DM, van Leeuwen MA, van Riel PL, van de Putte LB. Radiographic progression on radiographs of hands and feet during the first 3 years of rheumatoid arthritis measured according to Sharp’s method (van derHeijde modification). J Rheumatol. 1995;22:1792-1796.
- 9. Fuchs HA, Kaye JJ, Callahan LF, et al. Evidence of significant damage in rheumatoid arthritis within the first 2 years of disease. J Rheumatol. 1989;16:585-591.
- 10. Möttönen TT. Prediction of erosiveness and rate of development of new erosions in early rheumatoid arthritis. Ann Rheum Dis. 1988;47:648-653.
- 11. Boers M, Verhoeven AC, Markusse HM, et al. Randomised comparison of combined stepdown prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350:309-318.
- 12. Pincus T, Ferraccioli G, Sokka T, et al. Evidence from clinical trials and long-term observational studies that disease-modifying antirheumatic drugs slow radiographic progression in rheumatoid arthritis: updating a 1983 review. Rheumatology (Oxford). 2002;41:1346-1356.
- 13. Calgüneri M, Pay S, Caliskaner Z, et al. Combination therapy versus monotherapy for the treatment of patients with rheumatoid arthritis. Clin Exp Rheumatol. 1999;17:699-704.
- 14. Gerards AH, Landewé RB, Prins AP, et al. Cyclosporin A monotherapy versus cyclosporin A and methotrexate combination therapy in patients with early rheumatoid arthritis: a double blind randomised placebo controlled trial. Ann Rheum Dis. 2003;62:291-296.
- 15. Möttönen T, Hannonen P, Leirisalo-Repo M, et al; FIN-RACo trial group. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. Lancet. 1999;353:1568-1573.
- 16. Landewé RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347-356.
- 17. Anderson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000;43:22-29.
- 18. Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263-269.
- 19. Genovese MC, Bathon JM, Martin RW, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443-1450.
- 20. St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432-3443.
- 21. van der Heijde D, Klareskog L, Rodriguez- Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063-1074.
- 22. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
- 23. Hyrich KL, Lunt M, Watson KD, et al. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second antitumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007;56:13-20.
- 24. Biologic treatments for rheumatoid arthritis. American College of Rheumatology Web site. http://www.rheumatology.org/public/ factsheets/biologics.asp. Accessed May 4, 2007.
- 25. de Vries-Bouwstra JK, Dijkmans BA, Breedveld FC. Biologics in early rheumatoid arthritis. Rheum Dis Clin North Am. 2005;31:745-762.
- 26. Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50:1740-1751.
- 27. Setoguchi S, Solomon DH, Weinblatt ME, et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2757-2764.
- 28. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381-3390.
- 29. Puolakka K, Kautiainen H, Möttönen T, et al. Impact of initial aggressive drug treatment with a combination of disease-modifying antirheumatic drugs on the development of work disability in early rheumatoid arthritis: a five-year randomized followup trial. Arthritis Rheum. 2004;50:55-62.
- 30. van Aken J, van Dongen H, le Cessie S, et al. Comparison of long term outcome of patients with rheumatoid arthritis presenting with undifferentiated arthritis or with rheumatoid arthritis: an observational cohort study. Ann Rheum Dis. 2006;65:20-25.
- 31. Verpoort KN, van Dongen H, Allaart CF, et al. Undifferentiated arthritis-disease course assessed in several inception cohorts. Clin Exp Rheumatol. 2004;22(5 suppl 35):S12-S17.
- 32. Harrison BJ, Symmons DP, Brennan P, et al. Natural remission in inflammatory polyarthritis: issues of definition and prediction. Br J Rheumatol. 1996;35:1096-1100.
- 33. van der Helm-van Mil AH, le Cessie S, van Dongen H, et al. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum. 2007;56:433-440.
- 34. Emery P. Rheumatoid arthritis: not yet curable with early intensive therapy. Lancet. 1997;350:304-305.
- 35. Hoving JL, Buchbinder R, Hall S, et al. A comparison of magnetic resonance imaging, sonography, and radiography of the hand in patients with early rheumatoid arthritis. J Rheumatol. 2004;31:663-675.
- 36. Backhaus M, Burmester GR, Sandrock D, et al. Prospective two year follow up study comparing novel and conventional imaging procedures in patients with arthritic finger joints. Ann Rheum Dis. 2002;61:895-904.
- 37. Keen HI, Brown AK, Wakefield RJ, Conaghan PG. MRI and musculoskeletal ultrasonography as diagnostic tools in early arthritis. Rheum Dis Clin North Am. 2005;31:699-714.
- 38. Terslev L, Torp-Pedersen S, Savnik A, et al. Doppler ultrasound and magnetic resonance imaging of synovial inflammation of the hand in rheumatoid arthritis: a comparative study. Arthritis Rheum. 2003;48:2434-2441.
- 39. Szkudlarek M, Court-Payen M, Strandberg C, et al. Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum. 2001;44:2018-2023.
- 40. Stone M, Bergin D, Whelan B, et al. Power Doppler ultrasound assessment of rheumatoid hand synovitis. J Rheumatol. 2001;28:1979-1982.
- 41. Sugimoto H, Takeda A, Hyodoh K. MR imaging for evaluation of early rheumatoid arthritis. Semin Musculoskelet Radiol. 2001;5:159-165.
- 42. McQueen FM, Benton N, Perry D, et al. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1814-1827.
- 43. Ejbjerg B, Narvestad E, Rostrup E, et al. Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheum. 2004;50:1097-1106.
- 44. Ejbjerg B, McQueen F, Lassere M, et al. The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas: the wrist joint. Ann Rheum Dis. 2005;64(suppl 1):i23-i47.Â




