Article
Early Intervention with Secukinumab May Be Effective for Patients with Psoriasis
Author(s):
New data indicates that psoriasis patients who are naïve to biological therapies may benefit from early intervention with secukinumab, though further research is needed.
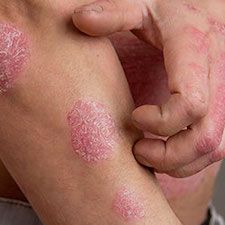
Early intervention with secukinumab for patients with psoriasis may be helpful for those with the skin disease including those who are bio-naïve, according to recent findings.1
There is evidence that the efficacy of secukinumab and the drug’s survival may both be strong in those previously non-exposed to biologic treatments.2 That said, real-world research and case series on long-term efficacy and survival of the treatment in naïve patients have been limited.
Consequently, this new multicenter study was done to analyze the long-term effectiveness and survival of the drug, and was authored by Francisco Javier Melgosa Ramos, MD, from the Department of Dermatology at the University Hospital Doctor Peset of Valencia in Spain.
“Our objective and primary endpoint was to analyze the long-term efficacy and survival of secukinumab for the treatment of psoriasis in a retrospective, multicenter study performed in a Spanish cohort of psoriatic patients naïve to biological therapies followed up to 8 years,” Ramos and colleagues wrote. “Secukinumab safety was evaluated as a secondary endpoint.”
Background and Findings
The investigators conducted the observational study across 8 Spanish hospitals to investigate the use of secukinumab in patients with psoriasis who had not previously received biological therapy. The team followed a daily practice cohort between January of 2014 and January of 2022, with a minimum follow-up period of 3 months and a maximum of 8 years.
The study participants initially received a weekly dose of 300 mg of secukinumab for 4 weeks, followed by monthly doses. However, some of the participants had their treatment regimen intensified or optimized based on the Physician Global Assessment (PGA) and disease control.
The study collected demographic data, information on previous nonbiological systemic therapies, and various study variables. The clinical response to the treatment was assessed by the research team using the Psoriasis Activity and Severity Index (PASI) throughout the period of their evaluation.
The investigators defined a complete response as reaching an absolute PASI score of ≤3 in plaque psoriasis or a PGA score of ≤1 in special localizations. The team also evaluated the safety of secukinumab and recorded any related side effects as a secondary endpoint.
Statistical analyses were done by utilizing the log-rank test to compare secukinumab survival time between different patient groups based on factors such as age, obesity, gender, and presence of psoriatic arthritis. Cox regression models were then utilized to explore the relationship between drug survival and patient characteristics.
The analyses also reported odds ratios (OR), with P < 0.05 being considered statistically significant. IBM SPSS v21.0 was used for the analyses.
The study ended up involving 128 individuals with moderate-to-severe cutaneous psoriasis who had not received biological therapies before. The investigators reported that around 92.2% of participants exhibited classical psoriasis plaques, while involvement in hard-to-treat areas was found to be less common.
It was reported that those included in the study were made up of 65 males and 63 females, with a mean age of 50.9 years. Approximately 47.2% of the participants were found to be obese.
The research team noted that psoriatic arthritis was detected in 18.8% of patients. The treatment response to secukinumab was evaluated at different follow-up periods, with a significant percentage of patients achieving low PASI scores.
The investigators also reported no occurrences of infections, malignancy, inflammatory bowel disease, or major adverse cardiovascular events associated with the treatment’s administration during the study period. No major differences were observed in terms of efficacy and survival rates based on factors such as BMI, gender, age, or the presence of psoriatic arthritis.
The research team noted that 86.6% - 100% of those ended up with a PASI score of 3 or lower, and 25% - 65.2% reached a PASI score of 0. The overall drug survival rate for secukinumab was also reported to be 81.9% over an average treatment exposure of 147.9 weeks.
“To conclude, as observed in our cohort, the high long-term efficacy and survival of secukinumab in bio-naïve patients and the low prevalence of arthritis and psoriasis-related comorbidities might suggest that managing severe psoriasis as a systemic disease, introducing advanced treatments early, could potentially modify the march of this disease, but further studies are needed,” they wrote.
References
- Melgosa Ramos, FJ, Mateu Puchades, A, Guillén Climent, S, et al. Long-term secukinumab efficacy and safety in bio-naïve patients with moderate-to-severe cutaneous psoriasis: a real-world retrospective noninterventional multicentric experience (128 patients). JEADV Clin Pract. 2023; 1– 7. https://doi.org/10.1002/jvc2.185.
- Daudén E, de Lima GPG, Armesto S, Herrera-Acosta E, Vidal D, Villarasa E, et al. Multicenter retrospective study of secukinumab drug survival in psoriasis patients in a daily practice setting: a long-term experience in Spain. Dermatol Ther. 2021; 11(6): 2207– 15.




