Article
Ertugliflozin Becomes Another Member of Growing SGLT-2 Inhibitor Class
Author(s):
The most recent approval in the class, ertugliflozin (Steglatro), is a product of the efforts of a duo of pharmaceutical giants, Pfizer and Merck.
THE SODIUM-GLUCOSE COTRANSPORTER-2 (SGLT-2) inhibitor class has grown to include almost 10 medications, with 4 approved for use by the US Food and Drug Administration (FDA) since 2011, providing much-needed additional options for the more than 30 million patients with type 2 diabetes (T2D) in the United States.1
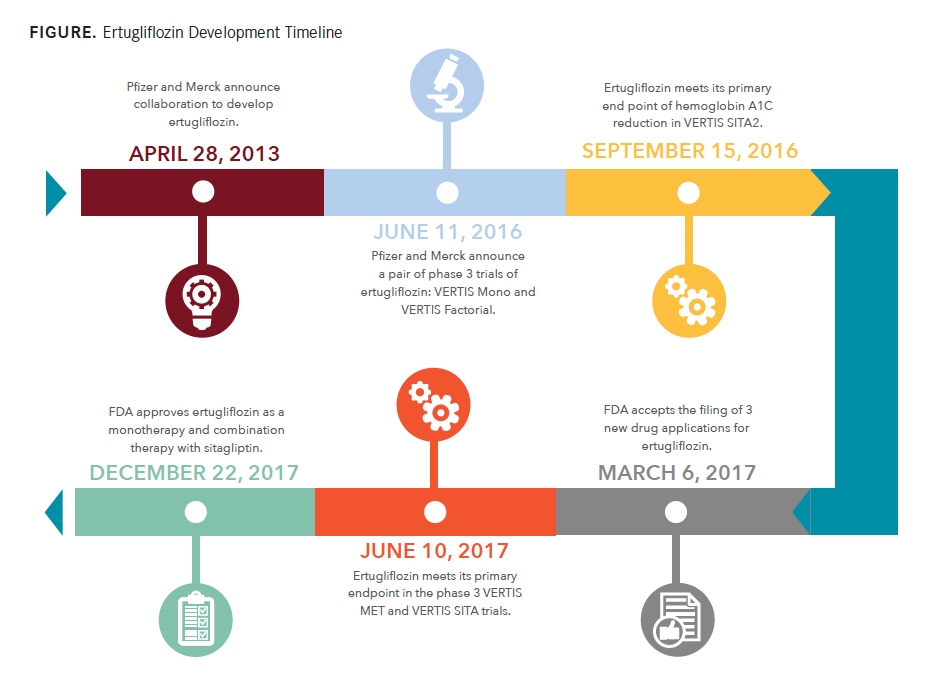
The most recent approval in the class, ertugliflozin (Steglatro), is a product of the efforts of a duo of pharmaceutical giants, Pfizer and Merck. FDA approval came in December 2017, as both a monotherapy and combination therapy with sitagliptin (Januvia, Merck) in doses of 5 and 15 mg for patients with T2D.
In the VERTIS MET trial, ertugliflozin showed blood glucose reductions of 0.7% and 0.9% for the 2 dose options, respectively, compared with 0% with placebo (P <.001).
The 26-week study evaluated the safety and efficacy of 5-and 15-mg ertugliflozin against placebo and metformin in adult patients with uncontrolled T2D currently on metformin monotherapy. Ertugliflozin significantly reduced patients’ fasting plasma glucose (FPG), body weight, systolic blood pressure, and diastolic blood pressure versus placebo.
Merck and Pfizer previously announced successfully met endpoints for the combination therapies in another phase 3 trial, VERTIS SITA, in June 2017. In this trial, 5- and 15-mg ertugliflozin with sitagliptin 100 mg were compared with placebo and sitagliptin. Patients on ertugliflozin reported greater reductions in A1C at the 5-mg (1.6%) and 15-mg (1.7%) doses versus placebo (0.4%; P <.001). The combination doses also significantly reduced FPG, body weight, and SBP in its patients versus placebo.
At the time of the announced study results, James Rusnak, MD, PhD, senior vice president and chief development officer of internal medicine at Pfizer Global Product Development, said the results “underscore the potential of ertugliflozin as an important therapeutic option” for adults with T2D.
“As the global burden of diabetes continues to rise, we are committed to meeting patients’ needs with additional treatment options to help manage their condition,” Rusnak said.
The only challenge that the therapy could face remains parallel with the rest of the SGLT-2 class. There are some fears of adverse effects related to the therapies. While an increased need to urinate and yeast infections (in women) are common in patients, there are bigger concerns.
In February 2017, the European Medicines Agency issued a warning of an increase in lower-limb amputations associated with SGLT-2 inhibitors. However, in May the FDA added that warning to only canagliflozin (Invokana, Janssen), as recent reevaluations of the EMPA-REG OUTCOME data have shown that the risk increase was not present with empagliflozin, leading researchers to believe it may be unique to the Janssen product.2
Additionally, the fear may be dispelled by the CVD-REAL 2 real-world data, presented at the American College of Cardiology’s 67th Scientific Sessions & Expo, which showed that SGLT-2 inhibitors were associated with a lower rate of death, hospitalization for heart failure, myocardial infarction, and stroke compared with other glucose-lowering drugs in patients with T2D.
Although that data were limited to dapagliflozin, empagliflozin, ipragliflozin, canagliflozin, tofogliflozin, and luseogliflozin, it is certainly a welcome sign for the class overall. “These findings suggest that the cardiovascular effects of SGLT-2 inhibitors may extend across patient ethnic and racial backgrounds, geographic regions, [and the cardiovascular] risk continuum,” Mikhail Kosiborod, MD, a cardiologist and clinical researcher at St. Luke’s Mid America Heart Institute and a professor of medicine at the University of Missouri-Kansas City, told MD Magazine®.
“SGLT-2 inhibition experience in real-world practice is still relatively short, and longer-term follow-up will be required to examine whether these effects are sustained over time,” Kosiborod added.
For ertugliflozin, information on real-world use will increase as the therapy became available to patients early this year. Clinical trials have shown it to result in significant A1C reductions when used alone or in combination, making it a versatile addition to a growing class of medications. Ultimately, the success of ertugliflozin, and the SGLT-2 class, will be determined in time.
“This is important, as A1C lowering is a key component of diabetes management and many of my adult patients may need multiple medications to help manage their condition,” Juan Pablo Frias, MD, president and principal investigator at the National Research Institute, in Los Angeles, said in a statement.
REFERENCES
National Diabetes Statistics Report, 2017. CDC website. cdc.gov/diabetes/pdfs/ data/statistics/national-diabetes-statistics-report.pdf. Published 2017. Accessed March 26, 2018.
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi: 10.1056/NEJMoa1504720.




