Article
Established and Novel Treatments for Lupus
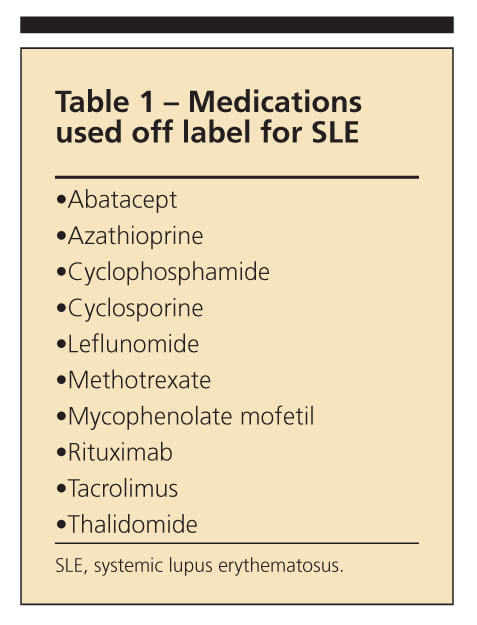
Although many medications are used for the management of systemic lupus erythematosus (SLE) and its complications, only aspirin, corticosteroids, and the antimalarial drug hydroxychloroquine (HCQ) are specifically approved by the FDA.1 Most other medications used for SLE treatment are commercially available off label (Table 1), usually borrowed from cancer or transplant regimens. In some cases, medications have been approved for a specific clinical manifestation seen in both idiopathic disease and SLE, such as bosentan for pulmonary hypertension.
Although many medications are used for the management of systemic lupus erythematosus (SLE) and its complications, only aspirin, corticosteroids, and the antimalarial drug hydroxychloroquine (HCQ) are specifically approved by the FDA.1 Most other medications used for SLE treatment are commercially available off label (Table 1), usually borrowed from cancer or transplant regimens. In some cases, medications have been approved for a specific clinical manifestation seen in both idiopathic disease and SLE, such as bosentan for pulmonary hypertension.
Many needs in lupus management remain unmet. These include medications with less toxicity or a better adverse effect profile that can prevent disease flares and accrual of damage resulting from the pathological changes of SLE itself or adverse effects of treatment (eg, corticosteroid-induced osteoporosis, diabetes mellitus, osteonecrosis). In addition, long-term complications, such as premature atherosclerosis, must be recognized and prevented.
Instead of targeting the entire immune system with the general immunosuppressive agents currently available, medications should be developed to target specific immunological dysfunction and specific disease features, and agents should be identified that have differential efficacy and toxicity in specific racial and ethnic groups. Identification of biomarkers to predict outcomes and drug efficacy also is essential.
In this article, we review the drugs currently available for SLE and the newer therapeutic agents in clinical trials for lupus in general or particular disease manifestations. We also discuss drugs used off label for the manifestations of SLE and management options for the complications of SLE.
FDA-approved medications for SLE
Corticosteroids often are used as the first-line agent for patients with most manifestations of SLE because of their powerful anti-inflammatory actions. The dose and duration vary with the severity of the features and rapidity of response. Instituting corticosteroid-sparing agents should be considered somewhat quickly, especially in the case of corticosteroid dependency or frequent exacerbations of disease activity.
HCQ was the last medication approved by the FDA for lupus in 1955. This drug has long been recognized as a valuable agent for managing fatigue, as well as cutaneous and musculoskeletal manifestations, and preventing lupus flares.2 More recently, evidence has shown that HCQ improves long-term survival of patients with SLE and protects against irreversible organ damage, thrombosis, and bone mass loss. Its adverse effect profile is quite favorable; the best-known complication, retinal toxicity, is rare, even with long-term use.
Despite Physicians' Desk Reference cautions about the use of HCQ in pregnancy, most rheumatologists and obstetricians are comfortable with patients continuing its use during pregnancy; there is virtually no evidence of fetal toxicity. In fact, HCQ improves pregnancy outcomes, with fewer lupus flares.2 Other antimalarial agents (eg, chloroquine and quinacrine) have poorer safety profiles, and closer monitoring is required.
NSAIDs are used for the treatment of patients with arthritis and arthralgia. These agents are particularly useful when the patient's initial workup is pending or the diagnosis is uncertain. However, potent NSAIDs (eg, ketorolac, indomethacin, diclofenac) are relatively contraindicated for patients with SLE because they increase the risk of acute renal failure and congestive heart failure. NSAIDs should be prescribed with particular caution in patients with lupus nephritis (LN) or renal impairment.
Medications in clinical trials
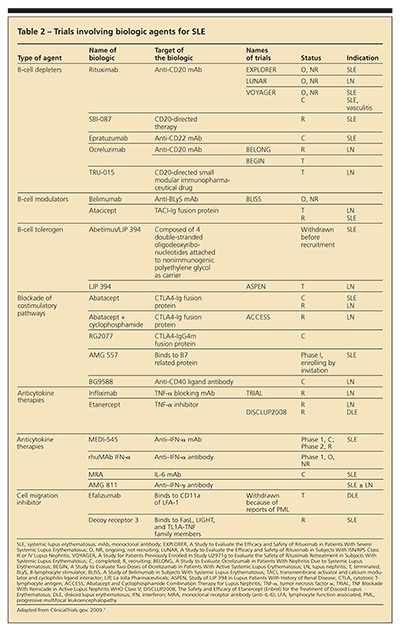
Biologic agents currently being studied for SLE have been designed to target various immunological processes, such as B-cell and T-cell functions, including production of cytokines (Table 2). These medications can lead to B-cell depletion, act as modulators and tolerogens, and block costimulatory and cytokine pathways. Although the biologic agents should work based on the known pathophysiology of SLE, this theory has not translated into efficacy in clinical trials; most have not met their study end points.
B-cell depleters include rituximab, epratuzumab, and ocrelizumab. Clinical trials with rituximab have not demonstrated efficacy in SLE or LN. However, case reports and series continue to be published about its use as "rescue therapy" in refractory severe SLE. Epratuzumab and ocrelizumab are fully human monoclonal antibodies to B-cell surface CD22 and CD20 antigens, respectively; epratuzumab, unlike rituximab, does not lead to complete B-cell depletion.
B-cell modulators are agents that bind to or inactivate B-cell surface receptors, which affect maturation, survival, and activity of B cells. The modulators target the ligands (B-lymphocyte stimulator [BLyS], B-cell–activating factor of the tumor necrosis factor [TNF] family [BAFF], and A proliferation inducing ligand [APRIL]) or their receptors (transmembrane activator and calcium modulator and cyclophilin ligand interactor [TACI], BAFF receptor, and B-cell maturation antigen [BCMA]).
Phase 2 trials for anti-BAFF agents and phase 2/3 trials for atacicept (TACI-Ig) are ongoing. The nephritis trial for atacicept was terminated early because of an unexpected incidence of infectious complications. Belimumab, the anti-BLyS monoclonal antibody, showed a reduction in anti-dsDNA titers correlating with SLE disease activity. Clinical trials of the B cell tolerogen abetimus sodium did not show efficacy in preventing LN flares.
Abatacept works by blocking the T-cell costimulatory pathway. Although this agent is FDA-approved for rheumatoid arthritis (RA), clinical trials of preventing flares of generalized SLE did not meet primary or secondary end points. Abatacept currently is being studied in patients with LN maintained on a background of mycophenolate mofetil (MMF) and corticosteroids. Trials of agents that block CD40:CD40L interaction were unsuccessful because of adverse thrombotic events.
Anti-interferon α (IFN-α) and IFN-γ agents are in phase 1, 2, and 3 trials. Anti–TNF-α agents have been used in patients who have mild SLE with arthritis as the predominant feature. Anti–TNF-α agents also are in trials for treatment of patients with LN and cutaneous lupus. However, given the case reports of drug-induced lupus in patients with RA who were treated with TNF-α inhibitors, patients with SLE need to be monitored closely when these drugs are administered.
Lupuzor is a novel immune modulator. All trials to date have been conducted in Europe and South America; phase 2a and 2b trials have been reported to meet their primary end points.
Other agents for lupus include cell migration inhibitors, such as efalizumab. After gaining approval for the management of plaque psoriasis and being studied for cutaneous lupus, efalizumab was withdrawn from the market because of reports of progressive multifocal leukoencephalopathy.
Managing organ-specific manifestations
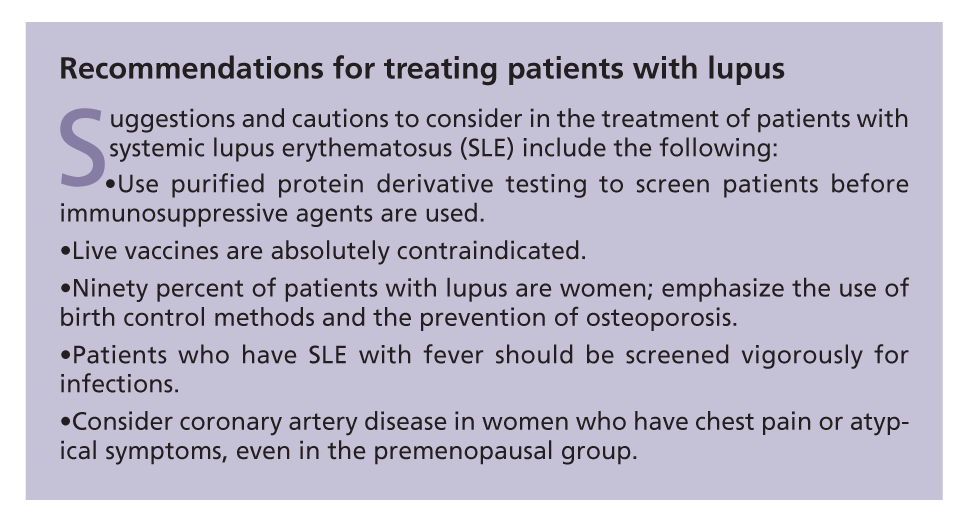
We have several suggestions for the management of organ-specific manifestations of SLE. They include the following:
•Corticosteroid-sparing agents. Azathioprine (AZA), MMF, methotrexate (MTX), and leflunomide all may be used as corticosteroid-sparing agents in SLE. Cyclosporine is rarely used, but it may be considered in patients who cannot tolerate other immunosuppressive agents. AZA is used as a corticosteroid-sparing agent for mild to moderate generalized lupus features, rash, and arthritis and as maintenance therapy for LN.
•Cutaneous lupus. The use of topical sunscreens with a high sun protective factor (SPF 45 to 60) should be emphasized to prevent flares. Antimalarials should be added to the treatment regimen as early as possible, along with oral corticosteroids, for moderate to severe cutaneous lupus. Milder lesions may be managed with topical agents, such as corticosteroid creams or tacrolimus. If other causes of the lesions are suspected, referral to a dermatologist for biopsy is needed.
Use of antimalarials other than HCQ, such as quinacrine and chloroquine, may be considered to control cutaneous lupus, but these agents carry an increased risk of retinal toxicity and rheumatologists do not use them frequently. If there is a poor response to the initial therapies, dapsone and colchicine may be added to the regimen.
Thalidomide has been very useful for refractory cutaneous lupus because of its antiangiogenic effects. However, this agent requires special regulation and close monitoring for pregnancy and neuropathy.
Therapeutic agents currently in trials for cutaneous lupus are topical pimecrolimus, betamethasone, thalidomide and, systemically administered, etanercept (a TNF-α blocker) and lenalidomide (a thalidomide derivative). MMF may be considered for refractory lesions.
•Musculoskeletal manifestations of SLE. Oral prednisone, antimalarials, NSAIDs, and celecoxib often are used as first-line agents for the management of synovitis in SLE. Disease-modifying antirheumatic drugs (eg, MTX and leflunomide) may be added in patients with arthritis in SLE, and they are useful in clinical practice. Abatacept and rituximab may be considered for the treatment of patients with refractory "rhupus," ie, patients with SLE who also have erosive, deforming arthritis or chronic synovitis. TNF-α inhibitors may be used in patients who have arthritis as their main manifestation, but caution needs to be exercised about the possibility of SLE flares in other organ systems.
•Lupus nephritis. The management of LN is divided into 2 phases, induction and maintenance. In the induction phase, a high dosage of prednisone equivalent (1 mg/kg/d) or pulse corticosteroids is used to rapidly suppress inflammation, along with other immunosuppressive drugs (eg, MMF). Corticosteroids should be tapered over the following several weeks to months, depending on the clinical response.
For the management of life-threatening manifestations, alkylating agents, such as cyclophosphamide (CYC), are being replaced by agents with more favorable adverse effect profiles (eg, MMF). In several randomized trials, MMF has been shown to be at least as effective as intravenous (IV) CYC for induction therapy of LN.3-5 MMF resulted in higher complete or partial remission rates in LN in African American and Afro-Caribbean minority populations; whites had similar responses to CYC and MMF.3-5
MMF generally is better tolerated than CYC, and most adverse effects are self-limited. However, questions remain about the appropriate dosage and duration of maintenance therapy after remission has been achieved.
AZA may be used for class II LN or for patients who cannot tolerate MMF, but it has not been shown to be as effective as MMF or IV CYC for induction regimens. During the maintenance phase, corticosteroids usually are discontinued while only the immunosuppressive agent-MMF or AZA-is continued.
Pulse intravenous corticosteroids, IV CYC, and IV gammaglobulin may be considered for patients with severe nephritis that is unresponsive to MMF and corticosteroids. Short-term hemodialysis may be an option for some patients in whom acute renal failure and fluid overload develop.
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) should be added and titrated as much as possible to aid in decreasing proteinuria, along with aggressive management of hypertension and dyslipidemia. Drugs in clinical trials for LN are abatacept, TRU-015, rituximab, fludarabine, ocrelizumab, infliximab, etanercept, AMG 811 (anti–IFN-γ), sirolimus, tacrolimus, and leflunomide.
•Neuropsychiatric manifestations of SLE (NPSLE). Life-threatening manifestations, such as transverse myelitis, may be managed with high-dose corticosteroids, plasmapheresis, and other immunosuppressive agents (eg, MMF and IV CYC). Although clinical trials have not shown efficacy with rituximab, this agent may be an alternative for patients with refractory disease.
Making evidence-based medicine recommendations for the management of NPSLE is difficult; it includes 19 entities (eg, transverse myelitis, seizures, and cerebritis). Some NPSLE manifestations, such as cognitive function, are difficult to evaluate. Patients with NP involvement are excluded from most clinical trials of therapeutic agents because they cannot sign the informed consent. One clinical trial of cognitive dysfunction involving memantine, which blocks the anti–N-methyl D-aspartate receptor, was completed recently. More efforts need to be made to include patients with NPSLE in clinical trials to improve their quality of life.
•Vascular manifestations. Vasculitis may be managed with the immunosuppressive agents described above for other manifestations in combination with corticosteroids. Thalidomide and rituximab also may be useful for the management of vasculitis in SLE.Raynaud phenomenon may be managed with calcium channel blockers, cilostazol, ticlopidine, topical nitrates, phosphodiesterase inhibitors (eg, sildenafil), and endothelin receptor antagonists (eg, bosentan) as needed to avoid digital gangrene. Patients should be advised to maintain a warm core body temperature, wear appropriate clothing, and avoid environmental triggers (eg, cigarette smoking).
•Pregnant patients with SLE. The only medication demonstrated to prevent flares during pregnancy is HCQ. Corticosteroids and AZA may be used for patients with active disease during pregnancy. AZA has been shown to be somewhat safe in pregnancy, although the dosage should be reduced in the last trimester because the fetal liver starts producing the enzyme inosinate pyrophosphorylase, which metabolizes the drug in this trimester. The success of pregnancy depends on the duration of well-controlled lupus before conception; longer duration correlates with better outcomes. If secondary antiphospholipid antibody syndrome is present with a history of previous pregnancy losses, heparin may be used for prophylaxis.
Medications used to prevent and manage complications of SLE
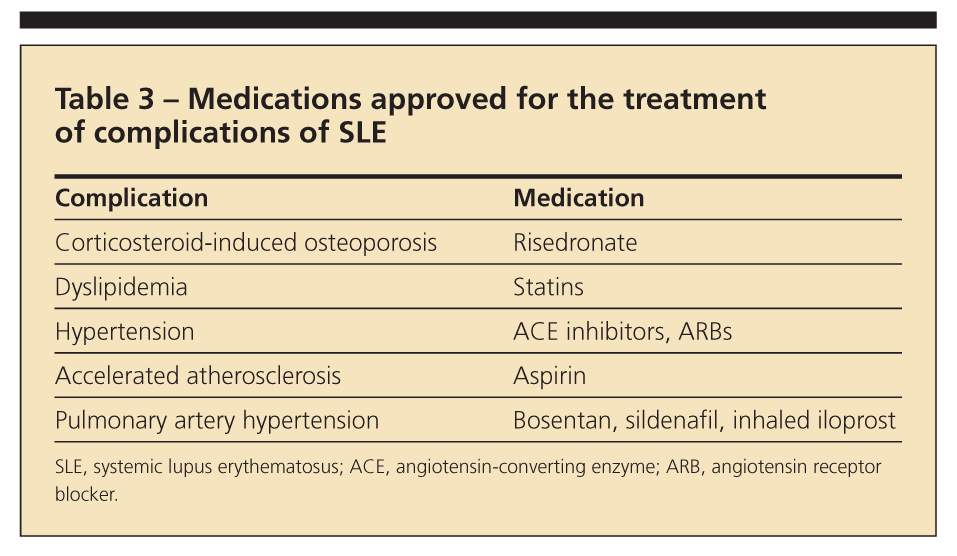
The leading causes of death in SLE are infections and atherosclerotic events (Table 3). Aggressive management of SLE has led to prolonged survival but with accrual of features of damage and more age-related comorbidities. Accelerated atherosclerosis-cardiovascular, cerebrovascular, or peripheral vascular disease-has been well documented in SLE. The active metabolite of MMF, mycophenolic acid, has antiproliferative effects on smooth muscle cells, mesangial cells, fibroblasts, and monocytes; these effects may translate into protection against atherosclerosis.6
Hypertension and hyperlipidemia should be managed aggressively. Apart from counteracting dyslipidemia, statins may have anti-inflammatory effects and are being explored in a clinical trial for the management of general lupus. ACE inhibitors and ARBs added to immunosuppressive agents help decrease proteinuria while they control hypertension. Agents that may be used for pulmonary arterial hypertension include calcium channel blockers, bosentan, sildenafil, and inhaled iloprost.
Corticosteroid-induced osteoporosis contributes to significant morbidity because of fractures. Vitamin D deficiency may accentuate this problem because of the use of sunscreens with a high SPF. Supplements of calcium and vitamin D should be the standard of care in all corticosteroid-treated patients.
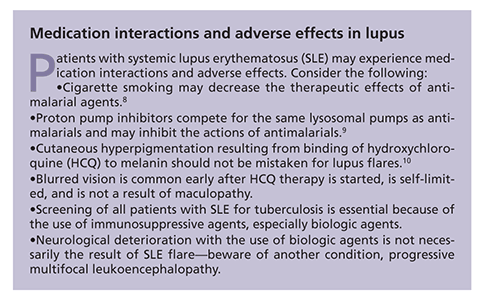
Bone density testing should be conducted every 2 years for screening. The addition of bisphosphonates should be considered to prevent bone resorption, but questions remain about their use in young childbearing women. For discussions of other important considerations, see the Boxes, "Recommendations for treating patients with lupus" and "Medication interactions and adverse effects in lupus" above.
Summary
Patients with SLE currently have an improved prognosis because of earlier diagnosis and aggressive management of active disease manifestations and complications. Because SLE is phenotypically heterogeneous, focused research is required on therapeutic agents for the management of organ-specific manifestations and for patient subsets based on individual characteristics. In recent clinical trials, most biologic agents have not shown superiority over the currently available immunosuppressive agents. However, with more than 200 clinical trials of SLE listed at the http://clinicaltrials.gov Web site,7 FDA-approved agents are likely to be available for treatment in the near future.
References:
References1. Rheumatology Information: Therapeutics for Systemic Lupus Erythematosus. http://www.fda.gov/Drugs/ResourcesForYou/HealthProfessionals/ucm107073.htm. Accessed July 16, 2009.
2. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2009; Jun 4. [Epub ahead of print].
3. Dooley MA, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int. 1997;51:1188-1195.
4. Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant. 2003;18:2039-2046.
5. Appel GB, Contreras G, Dooley MA, et al; Aspreva Lupus Management Study Group. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103-1112.
6. Dubus I, L’Azou B, Gordien M, et al. Cytoskeletal reorganization by mycophenolic acid alters mesangial cell migration and contractility. Hypertension. 2003;42:956-961.
7. ClinicalTrials.gov. http://clinicaltrials.gov. Accessed July 16, 2009.
8. Kreuter A, Gaifullina R, Tigges C, et al. Lupus erythematosus tumidus: response to antimalarial treatment in 36 patients with emphasis on smoking. Arch Dermatol. 2009;145:244-248.
9. Namazi MR. The potential negative impact of proton pump inhibitors on the immunopharmacologic effects of chloroquine and hydroxychloroquine. Lupus. 2009;18:104-105.
10. Puri PK, Lountzis NI, Tyler W, Ferringer T. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35:1134-1137.




