Article
Evaluating shin pain in active patients
Shin pain is a common complaint in runners and otheractive patients. Making a diagnosis can be difficult because the differentialis broad and symptoms may overlap. Palpation is an importantpart of the physical examination. The primary presentingsymptom in medial tibial stress syndrome, or "shin splints," is painlocalized to the medial border of the distal third of the tibia. Relativerest eliminates the inciting activity. The most obvious examinationfinding in tibial stress fractures is localized bony tenderness; triplephasebone scanning is the gold standard in making a diagnosis.High-risk fractures require aggressive management. The mainstay ofdiagnosis of chronic exertional compartment syndrome is measurementof resting and postexercise compartment pressures. Treatmentmay be conservative or surgical. (J Musculoskel Med. 2008;25:138-148)
ABSTRACT: Shin pain is a common complaint in runners and other active patients. Making a diagnosis can be difficult because the differential is broad and symptoms may overlap. Palpation is an important part of the physical examination. The primary presenting symptom in medial tibial stress syndrome, or "shin splints," is pain localized to the medial border of the distal third of the tibia. Relative rest eliminates the inciting activity. The most obvious examination finding in tibial stress fractures is localized bony tenderness; triple-phase bone scanning is the gold standard in making a diagnosis. High-risk fractures require aggressive management. The mainstay of diagnosis of chronic exertional compartment syndrome is measurement of resting and postexercise compartment pressures. Treatment may be conservative or surgical. (J Musculoskel Med. 2008;25:138-148)
Shin pain is a common complaint in active patients, particularly those who participate in running sports. When a patient presents with shin pain, the physician should be able to recognize the common presentations to direct the workup, treatment, and rehabilitation appropriately. In addition, the complaint should be evaluated properly to avoid missing a potentially more serious condition.
The main causes of shin pain are medial tibial stress syndrome (MTSS), or "shin splints"; tibial stress fracture; and chronic exertional compartment syndrome (CECS).1 Less common causes include referred pain from the lumbosacral spine, neurovascular pathology, and bone tumors.
Many of the disorders may be designated as overuse injuries.The contributing factors include too much activity, inadequate strength and flexibility, muscle imbalance, inappropriate running surface and terrain, lower extremity malalignment, and use of inappropriate footwear.2,3
Making a diagnosis can be difficult because the differential is broad, symptoms may overlap, and atypical presentations may occur. In this article, we discuss the history taking, physical examination, and clinical features of the main and less common causes of shin pain to provide an efficient evaluation. We also describe approaches to effective treatment.
HISTORY
A thorough history taking is essential. The physician must ascertain the circumstances surrounding the injury, the presence of any specific trauma, and a clear description of the symptoms. Obtaining a detailed training history is useful; attention should be paid to any recent changes in mileage, footwear, intensity, or running surfaces. The focus should be on pain onset, the relationship of pain to activity, associated symptoms, and any alleviating or aggravating factors.1,4
PHYSICAL EXAMINATION
Begin the physical examination by observing the patient and making note of his or her gait and hip, knee, and ankle motion. Palpation is important at the site of maximal tenderness; the entire tibia and fibula; and the surrounding tissues, including the medial "gutter," where the calf musculature attaches to the medial tibia. Tenderness over the medial gutter probably is related to MTSS; focal bony tenderness is indicative of a stress fracture.
Assess flexibility of the patient's heel cords, range of motion and strength at the ankle, and neurovasculature of the extremity. Tightness of heel cords and decreased range of motion at the ankle and subtalar joints can predispose persons to overuse injuries, such as MTSS and stress fractures. Sensory deficits and weakness not limited by pain point to CECS, nerve entrapment, or referred lumbosacral pathology.
Attempt to reproduce symptoms by asking the patient to perform active and passive movements, resisted movements, and functional tests. Tests may include single-leg toe raises, the hop test (in which the patient hops on 1 leg), and even observed running for a few minutes on a treadmill or outdoors.1,4 Single-leg toe raises stress the musculotendinous structures; pain can be elicited if there is MTSS or muscle overuse. Results of the hop test are positive if pain is reproduced with hopping on 1 leg and may indicate a stress fracture.
CAUSES OF SHIN PAIN
Medial tibial stress syndrome
The term "shin splints" was once used as the primary diagnosis for most exercise-induced lower leg pain. Now it usually is used as a descriptive term for exertional shin pain rather than a diagnostic term-it does not define the location or source of the pain. The terms currently used to describe shin splints more specifically include "MTSS," "medial periostalgia," "medial tibial periostitis," and "traction periostitis." MTSS is twice as common in women as in men.5-7
MTSS is thought to be an overuse injury of the muscle-tendon units of the deep posterior compartment, as well as periosteal inflammation of the medial tibia (Figure 1).5 The exact pathophysiology is not well known. Some authors maintain that MTSS is caused by chronic traction on the periosteum at the periosteal-fascial junction. Two muscles have been implicated as the cause of this traction: the tibialis posterior and the soleus.7,8
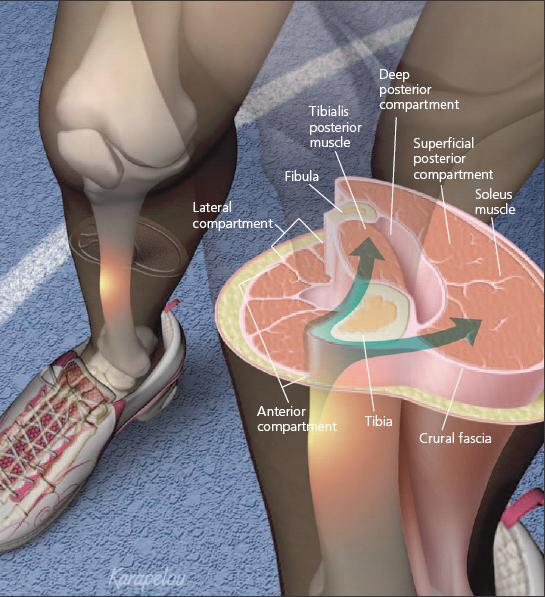
Figure 1
- Physicians should be able to recognize the common presentations of shin pain to direct the workup, treatment, and rehabilitation. Medial tibial stress syndrome (MTSS), or "shin splints," is thought to be an overuse injury of the muscle-tendon units of the deep posterior compartment that may involve periosteal inflammation of the medial tibia. MTSS may be caused by chronic traction (arrows) on the periosteum at the periostealfascial junction; the tibialis posterior and soleus muscles have been implicated as the cause.
There probably is no single cause of MTSS. A variety of factors may contribute to its development, including the running surface; the level of the athlete's conditioning; an increase in activity level; footwear; and abnormal biomechanics, which may include hyperpronation and heel rather than forefoot running.
Clinical features. The primary presenting symptom of MTSS is pain localized to the medial border of the distal third of the tibia. As in other overuse injuries, the pain initially is mild, develops toward the end of exercise, and is relieved with rest. If the athlete continues to exercise, the pain increases in intensity and begins to occur earlier or may occur with walking or at rest.
On physical examination, a diffuse area of tenderness often is found along the medial border of the distal tibia and along the medial gutter.7 Pain may be elicited with dorsiflexion or plantar flexion of the foot against resistance. In more severe cases, mild induration and swelling over the involved area may be noticed; in some chronic cases, thickening or nodularity may be palpated.5,6 The results of neurovasculature assessment are normal.
A diagnosis of MTSS usually is made with a thorough history and physical examination. However, imaging studies are ordered if the diagnosis is unclear and the clinician wants to rule out occult fracture, stress fracture, or bone tumor. Most patients with MTSS have normal radiographic findings.
Triple-phase bone scanning (TPBS) is highly sensitive in detecting bone stress lesions; this study often is the next to be ordered. TPBS usually shows superficial periosteal hyperconcentration or patchy areas of increased uptake along the medial border of the tibia.7,9
MRI may be used in evaluation of shin pain; it is similar to TPBS in its sensitivity and specificity.10 This alternative offers the benefits of having no radiation and providing additional evaluation of soft tissue structures. Therefore, although MRI is not required, it probably is the study of choice to make the diagnosis.
Treatment. Management of MTSS includes the same principles that are involved in management of other overuse injuries. Relative rest eliminates the inciting activity, allowing the inflamed muscle-tendon units or periosteum to heal; the patient also may engage in cross training (eg, bicycling, swimming, and pool running) to maintain fitness. The required duration of rest varies with the severity of the injury; it may be as short as a few days or as long as a few weeks.
During the acute phase, NSAIDs and anti-inflammatory modalities (eg, ice massage and iontophoresis) may help reduce inflammation and pain. Iontophoresis is a therapeutic means of introducing a topically applied corticosteroid, such as dexamethasone, into deeper soft tissue structures via a small electric current. A neoprene sleeve worn on the lower leg also may provide pain relief.
After the acute phase, a strengthening and conditioning program should be started. The focus should be on the muscles of the anterior and posterior compartments. Flexibility exercises that focus on the hamstrings, quadriceps, and calf muscles should be encouraged.2,5
Prevention involves avoidance of sudden increases in training mileage, frequency, and intensity; correction of abnormal biomechanics; and selection of appropriate footwear based on foot type and biomechanics.11 Many clinicians think that there is a continuum of injury from MTSS to stress fracture; if not managed adequately, MTSS could develop into a stress fracture.
Stress fracture
A stress fracture is defined as a partial or complete bone fracture that results from repetitive stress at a level lower than that required to fracture bone in a single loading. Stress fractures result from an imbalance between bone resorption and bone replacement that leads to weakening of the bone and subsequent fracture.1
Stress fractures occur more often in women than in men and account for about 10% of all injuries in athletes.2 They are more likely to occur in sports that involve extensive running or jumping and in patients who have had a previous stress fracture.2 The incidence probably is increased in athletes who have the female athlete triad (disordered eating, absent menses, and osteoporosis).1 There is a complex interaction between extrinsic factors (eg, training errors and training surface) and intrinsic factors (eg, endocrine elements, bone density, biomechanics, nutrition, and physical fitness).12
Most stress fractures involve the lower extremities; the tibia accounts for almost half these injuries. Most tibial stress fractures occur over the posteromedial border of the distal third. In 17% of cases, the injury is bilateral.12,13 Fibula stress fractures are less common; they also usually occur in the distal third.2
Clinical features. A patient who has a stress fracture typically presents with the complaint of a gradual onset of pain related to his activity. The pain classically is relieved by rest, but it may linger after cessation of activity.1,13 If the patient does not modify his activity, the pain may progress and become constant; night pain also may occur. All patients should be questioned about trauma, changes in their training program, footwear, terrain, nutrition and, for women, their menstrual history.12
The most obvious physical examination finding is localized bony tenderness.The hop test result will be positive, but this is not a very specific test. Mild swelling may be present, as well as pain with percussion or with torsional or bending stress. Rarely, there may be localized swelling, erythema, and warmth.12-14
Some authors making the diagnosis have found it useful to apply a vibrating tuning fork to the area of maximal tenderness and elicit increased pain. Others have advocated applying therapeutic ultrasonography over the painful area; however, studies have shown that this modality has a very low sensitivity in stress fracture detection, as well as being costly, making its use inappropriate.15,16
Imaging is the next step in evaluation for suspected stress fracture. The sensitivity and specificity for detecting stress fracture on plain x-ray films are 26% and 100%, respectively, varying with the injury age and severity.9 The results may be falsely negative for up to 3 months after symptom onset, and any abnormalities (eg, fracture lines, lucency, periosteal thickening, and early callus formation) typically are late findings unlikely to be present if symptoms have been present for less than 3 weeks (Figure 2). Only 50% of stress fractures are seen on plain radiographs.2,12
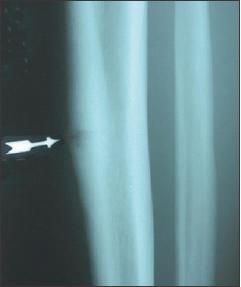
Figure 2
- The results of radiography in patients with early-stage tibial stress fractures typically are normal. One type of tibial stress fracture, that of the anterior cortex (arrow), is prone to acute fracture, prolonged healing, and nonunion.
TPBS currently is the gold standard in making a diagnosis of stress fractures. It should be ordered if radiographic results are normal but there still is a high level of suspicion for stress fracture. Sensitivity approaches 100%, and specificity is 76%.9 A normal TPBS result effectively rules out a stress fracture or other active bony process. The bone scan usually shows dense focal increased tracer concentration at the site of the fracture.9
MRI has been found to have comparable sensitivity and superior specificity. It has the additional advantages of identifying the exact location of the stress fracture and showing concomitant soft tissue damage.
CT scanning plays a limited role in the diagnosis of stress fractures. It is less sensitive than TPBS and plain radiographs. Its main advantage over MRI lies in the diagnosis of a longitudinal tibial fracture, which an MRI often does not reveal.14 CT also helps differentiate stress fracture from an osteoid osteoma-both may be "hot" on bone scan, show edema on MRI, and have sclerosis on radiographs.13
Treatment. The time from diagnosis of stress fracture to full return to activity varies with the fracture site, duration of symptoms, and severity of the lesion. Stress fractures may be managed effectively by stopping the offending activity and allowing bone formation to "catch up."12
Most tibial stress fractures that have a somewhat brief duration of symptoms heal without complication, allowing for full return to activity within 6 to 12 weeks.7 Bony tenderness usually abates after 4 to 8 weeks, after which the patient may return to activity gradually.2
Low-risk fractures, including those of the posterior or medial tibia and distal fibula, may be managed conservatively. The initial treatment focuses on pain control with ice and relative rest with weight bearing as tolerated. Whether NSAIDs may actually inhibit stress fracture healing is a subject of debate.17
Pain is a useful guide for assessing healing. If pain is severe, the athlete may need no weight bearing or partial weight bearing with crutches for 7 to 10 days. Several studies have shown that wearing a high pneumatic leg brace helps decrease pain and time to return to activity.2,7,12
Bone stimulator use in the management of tibial stress fractures has not been well studied, but it may be a consideration for fractures that do not heal after conservative measures are used. An increase in calcium and vitamin D intake should be advised to help healing. Muscle-strengthening and cross-training programs should be prescribed to help patients maintain strength and cardiovascular fitness.
High-risk fractures, including those of the anterior mid tibia, longitudinal tibia, and medial and lateral malleoli, require more aggressive management, such as non–weight-bearing immobilization and, in some cases, internal fixation. Patients with these fractures may expect a much longer recovery time and probably should be monitored by a physician who is quite comfortable with fracture management.1
Delayed union, or "dreaded blackline," stress fractures of the anterior mid tibia account for about 5% of tibial stress fractures. These injuries are more common with activities that require repetitive jumping.18 They have a poor prognosis and a propensity to progress to a complete fracture, probably because there is constant tension from the posterior calf musculature and a relative hypovascularity of the anterior tibia.
Conservative management involves rest and all the modalities listed above. Patients should not return to activity until there is evidence of cortical bridging on radiography. If there is no evidence of healing after 4 to 6 months, a trial of bone stimulator or surgical management should be considered, including focal drilling, bone grafting and intramedullary nailing. Full return to activity may take up to a year.7,18 Prevention of stress fractures involves educating patients, coaches, and trainers about proper training regimens, avoidance of sudden increases in workout intensity, wearing of proper shoes (with and without orthoses) to correct biomechanical abnormalities, good nutrition, and the importance of adequate rest and recovery time.13
Chronic exertional compartment syndrome
This pain syndrome results from a noncompliant osseofascial compartment that is unresponsive to the expansion of muscle volume that occurs with exercise.The exact cause of pain is not known, but it is thought to be relative ischemia of the involved compartment musculature or increased sensitivity of pain receptors in the compartment.
CECS may occur in the upper or lower extremity, but about 95% of cases occur in the lower leg. The condition affects mostly persons involved in running activities, but it may be found in nonathletes. The incidence is almost equally divided between men and women. CECS differs from acute compartment syndrome, which often is induced by trauma and requires urgent surgical attention to avoid irreversible tissue damage.19
The lower leg is composed of 4 compartments: anterior, lateral, superficial posterior, and deep posterior. Each compartment contains a major nerve, and both the anterior and deep posterior compartments house a major blood vessel. Most authors think that the anterior compartment is most affected, although some claim that it is the lateral compartment.19
Several factors are thought to play a role in the increase in compartment pressures, including compartments being enclosed by inelastic fascial sheaths, increased volume of skeletal muscle during exercise, muscle hypertrophy in response to exercise, and dynamic contraction factors in the gait cycle.2 During exercise, muscle fibers may swell up to 20 times their resting size, leading to an increase in their volume and weight.19 This increase causes a rise in compartment pressure and, probably, a decrease in blood flow through the muscle.
Clinical features. The patient usually presents with complaints of pain with exertion over the involved compartment.The pain typically is described as a dull ache, a tightness or cramping, or a squeezing ache.The onset and the degree of pain often become predictable and reproducible because the pain occurs at a well-defined point during exercise. Symptoms usually resolve after cessation of activity but may persist. If ignored, symptoms may worsen progressively over weeks or months. The athlete also may complain of transient numbness, tingling, or weakness of the involved extremity. Most cases are bilateral.2,19,20
The results of physical examination of the at-rest athlete typically are normal. If the symptoms are unilateral, muscle atrophy may be present. Occasionally, muscle hypertrophy and tenderness to palpation of the affected compartment are noted. Muscle hernia may be seen in 20% to 60% of cases.20 If the athlete is examined immediately after exercise, he may display objective findings of muscle weakness or decreased sensation in the distribution of the neuromuscular units of the involved compartment.
A diagnosis of CECS rarely can be made by examination alone.The mainstay of diagnosis is measurement of resting and postexercise compartment pressures, which may be accomplished with a needle manometer or wick catheter.
The generally accepted criteria for the diagnosis of CECS are shown in Table 1. Pressure measurements are taken pre-exercise and then at 1 and 5 minutes postexercise. One or more of the pressure criteria must be met to make the diagnosis2; some authors suggest that all the criteria should be met. The invasive nature of the testing for CECS has led to increased interest in noninvasive diagnostic modalities, including near infrared spectroscopy, MRI, thallium-201 SPECT scanning,and technetium-99m methoxyisobutyl isonitrile perfusion imaging.2,19
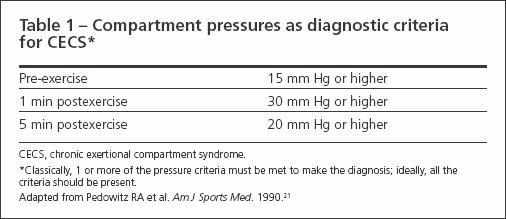
Table 1
Treatment. Although treatment of patients with CECS may be conservative or surgical, only surgery has been found to be curative. Conservative treatment may include avoidance of the offending activity and physical therapy (eg, ultrasonography, deep friction massage, and electrostimulation).
If conservative therapy has not succeeded after 6 to 12 weeks, surgical treatment should be pursued. 2 The most frequently performed procedure is a subcutaneous fasciotomy, although some authors advocate an open fasciectomy or a combination of the two. Reports of symptom improvement after surgery range from 50% to 100%.19,20 Full return to activity may be possible 4 to 12 weeks after surgery.
Other causes
Less common causes of shin pain should not be overlooked. Referred pain from the lumbosacral spine (eg, with radiculopathy or spinal stenosis) may present as shin pain. Focal nerve entrapment of the superficial peroneal nerve, deep peroneal nerve, or sural nerve may cause pain. A review of the differential diagnosis of shin pain is shown in Table 2.
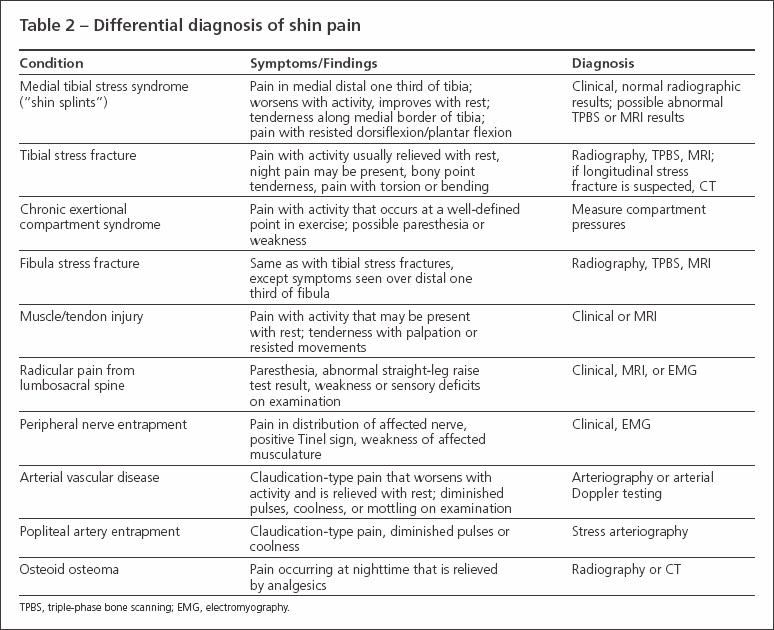
Table 2
References:
References
- Fredericson M, Wun C. Differential diagnosis of leg pain in the athlete. J Am Podiatr Med Assoc. 2003;93:321-324.
- Wilder RP, Sethi S. Overuse injuries: tendinopathies, stress fractures, compartment syndrome, and shin splints. Clin Sports Med. 2004;23:55-81.
- Taunton JE, Ryan MB, Clement DB, et al. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36:95-101.
- Brukner P, Khan K, Bradshaw C. Shin pain. In: Brukner P, Khan K, eds. Clinical Sports Medicine. 2nd ed. Sydney, Australia: McGraw-Hill; 2001:508-523.
- Moore MP. Shin splints diagnosis, management, prevention. Postgrad Med. 1988;83:199- 210.
- Abramowitz AJ, Schepsis A, McArthur C. The medial tibial syndrome: the role of surgery. Orthop Rev. 1994;23:875-881.
- Brukner P. Exercise-related lower leg pain: bone. Med Sci Sports Exerc. 2000;32 (3 suppl):S15-S26.
- Michael RH, Holder LE. The soleus syndrome: a cause of medial tibial stress (shin splints). Am J Sports Med. 1985;13:87-94.
- Shikare S, Samsi AB, Tilve GH. Bone imaging in sports medicine. J Postgrad Med. 1997;43: 71-72.
- Batt ME, Ugalde V, Anderson MW, Shelton DK. A prospective controlled study of diagnostic imaging for acute shin splints. Med Sci Sports Exerc. 1998;30:1564-1571.
- Thacker SB, Gilchrist J, Stroup DF, Kimsey CD. The prevention of shin splints in sports: a systematic review of literature. Med Sci Sports Exerc. 2002;34:32-40.
- Tuan K, Wu S, Sennett B. Stress fractures in athletes: risk factors, diagnosis, and management. Orthopedics. 2004;27:583-593.
- Spitz DJ, Newberg AH. Imaging of stress fractures in the athlete. Radiol Clin N Am. 2002; 40:313-331.
- Roebuck JD, Finger DR, Irvin TL. Evaluation of suspected stress fractures. Orthopedics. 2001;24:771-773.
- Boam WD, Miser WF, Yuill SC, et al. Comparison of ultrasound examination with bone scintiscan in the diagnosis of stress fractures. J Am Board Fam Pract. 1996;9:414-417.
- Romani WA, Perrin DH, Dussault RG, et al. Identification of tibial stress fractures using therapeutic continuous ultrasound. J Orthop Sports Phys Ther. 2000;30:444-452.
- Wheeler P, Batt ME. Do non-steroidal antiinflammatory drugs adversely affect stress fracture healing? A short review. Br J Sports Med. 2005;39:65-69.
- Batt ME, Kemp S, Kerslake R. Delayed union stress fractures of the anterior tibia: conservative management. Br J Sports Med. 2001;35:74-77.
- Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11:268-276.
- Shah SN, Miller BS, Kuhn JE. Chronic exertional compartment syndrome. Am J Orthop. 2004;33:335-341.
- Pedowitz RA, Hargens AR, Mubarak SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18:35-40




