Article
Experts React to Upadacitinib Approval for Ulcerative Colitis
Author(s):
Upadacitinib is now approved for ulcerative colitis, atopic dermatitis, and psoriatic arthritis.

The approval of upadacitinib (RINVOQ) could represent a game changer for patients with ulcerative colitis.
While the drug was previously approved by the US Food and Drug Administration (FDA) for atopic dermatitis and psoriatic arthritis, the March 16 approval represents the first time it was earmarked for a disease in gastroenterology.
The selective Janus kinase (JAK) inhibitor is now added to an ever growing stable of treatment options for patients with inflammatory bowel disease (IBD), particularly ulcerative colitis.
"Ulcerative colitis patients live with unpredictable symptoms such as increased stool frequency and bleeding, which can make daily activities difficult," said Maria T. Abreu, MD, Professor of Medicine, Professor of Microbiology and Immunology, University of Miami Miller School of Medicine and Director, Crohn's & Colitis Center, University of Miami Health System, in a statement. "In clinical trials, RINVOQ showed its ability to rapidly control symptoms in just eight weeks for many patients and sustained responses at one year. I believe these types of improvements can make a positive difference for my patients."
Previously, Silvio Danese, MD, lead study investigator of the U-ACCOMPLISH trial and head of the Inflammatory Bowel Diseases Centre at Humanitas Research Hospital, spoke on the promise of upadacitinib as a treatment for ulcerative colitis.
"People living with moderate to severe ulcerative colitis continue to suffer from the significant burden of this disease," said Silvio Danese, MD, lead study investigator and head of the Inflammatory Bowel Diseases Centre at Humanitas Research Hospital, in a statement in 2021. "I am very impressed with the consistent results seen in both ulcerative colitis induction studies, suggesting that upadacitinib could be a potential new treatment option for patients."
With the treatment now approved, several well-known gastroenterologists have taken to Twitter how the availability of upadacitinib could be crucial in treating their patients.
David T. Rubin, MD, University of Chicago Medicine Inflammatory Bowel Disease Center:
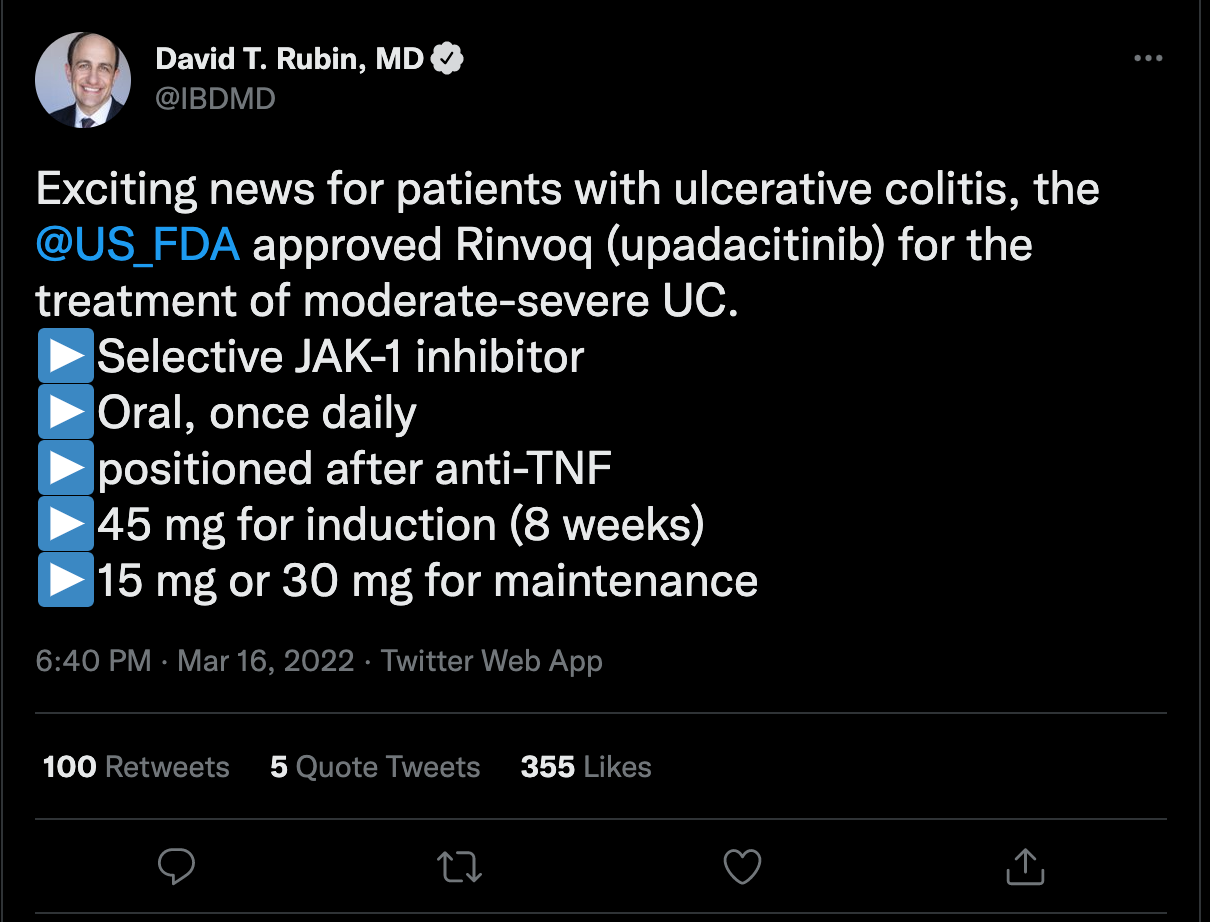
Shubha Bhat, PharmD, Cleveland Clinic:
Peter Higgins, MD, PhD, the director of the IBD program at the University of Michigan’s Department of Internal Medicine: