Article
FDA Clears Reader for Abbott FreeStyle Libre 3 System
Author(s):
Announced on April 14, the clearance allows for FreeStyle Libre 3 users to obtain real-time glucose readings on a large, easy-to-see screen directly from their back of arm sensors.
FreeStyle Libre 3 Reader and Sensor
Credit: Abbott
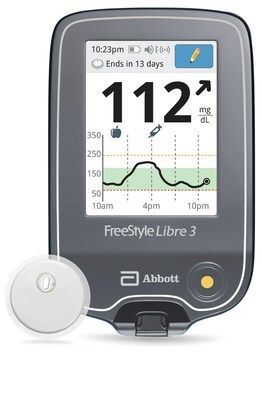
The US Food and Drug Administration has granted clearance to a reader for Abbott’s FreeStyle Libre 3 integrated continuous glucose monitoring system (CGM), according to an announcement from Abbott.
Announced on April 14, 2023, the standalone reader, which will use a rechargeable lithium-ion battery, displays real-time glucose readings allowing people to manage their diabetes on a large, easy-to-see screen.
"Our customers all over the world consistently tell us how our FreeStyle Libre technology has made an enormous, positive impact on their health and quality of life – they spend less time worrying and more time living," said Jared Watkin, senior vice president for Abbott's diabetes care business.1 "The FreeStyle Libre 3 reader provides more choice to people living with diabetes to have access to lifesaving technology that is smaller and easier to use and comes without the high-cost burdens of other systems."
According to Abbott, the FreeStyle Libre portfolio is the most prescribed sensor-based glucose monitoring system in the world and has been used by more than 4.5 million worldwide. Approved in May 2022, the Abbott FreeStyle Libre 3 is billed by the company as the world’s smallest, thinnest, and most accurate 14-day glucose sensor. The integrated CGM boasts an overall mean absolute relative difference of 7.9% and is the size of 2 stacked US pennies.2
With the clearance of the Abbott FreeStyle Libre 3 standalone reader, which users can use the small handheld device to get real-time glucose readings directly from the Abbott sensor, Abbott pointed out they are working with the Centers for Medicare and Medicaid Services to include the FreeStyle Libre 3 system to Medicare’s listed of covered systems.1
The clearance comes less than 2 weeks after Abbott readers made headlines for a different reason. On April 6, the FDA announced a Class I recall of Abbott FreeStyle readers for risk of overheating. In the recall announcement, the FDA noted Abbott had received 206 taproots related to the safety of the readers, which the company pointed out was representative of only 0.0017% of readers sold worldwide. In their own statement, Abbott noted the recall is not a physical recall and the company recommends avoiding use of third-party charges, exposure to liquid, introduction of foreign material to ports, and improper storage to minimize risk of overheating.3
“While rechargeable lithium-ion batteries are generally safe when used properly, they may present a fire hazard if they are damaged or used improperly," said Abbott in their official statement.3 "We’ve been communicating with people who use our FreeStyle Libre family of readers since February to ensure they know how to safely store, charge and use our readers and have set up a website www.FreeStyleBattery.com where they can learn more. This notice does not impact people who do not own a reader or only use FreeStyle Libre portfolio sensors with our mobile apps.”
As it pertains to Abbott pointed out steps are available on www.FreeStyleBattery.com provide guidance on how to properly store, charge and use a reader and its accompanying USB cable and power adapter.3
References
- FDA clears reader for Abbott's Freestyle Libre® 3 system. Abbott MediaRoom. https://abbott.mediaroom.com/2023-04-14-FDA-Clears-Reader-for-Abbotts-FreeStyle-Libre-R-3-System#assets_2429_124594-111. Published April 14, 2023. Accessed April 17, 2023.
- Abbott's Freestyle Libre® 3 receives U.S. FDA clearance - features world's smallest, thinnest and most accurate 14-day glucose sensor. Abbott MediaRoom. https://abbott.mediaroom.com/2022-05-31-Abbotts-FreeStyle-Libre-R-3-Receives-U-S-FDA-Clearance-Features-Worlds-Smallest,-Thinnest-and-Most-Accurate-14-Day-Glucose-Sensor. Published May 31, 2022. Accessed April 17, 2023.
- Campbell P. FDA issues class I recall of Abbott Freestyle Readers for risk of overheating, fire. HCP Live. https://www.hcplive.com/view/fda-issues-class-i-recall-of-abbott-freestyle-readers-for-risk-of-overheating-fire. Published April 6, 2023. Accessed April 17, 2023.





