Article
Gradual Glucocorticoid Withdrawal Does Not Increase Flares in Systemic Lupus Erythematosus
Author(s):
Glucocorticoid withdrawal over a prolonged period of time did not increase clinical flares or damage for patients with inactive systemic lupus erythematosus.
Steadily lowering glucocorticoid doses over a prolonged period of time did not increase clinical flares or damage at the end of 2 years in patients with inactive systemic lupus erythematosus (SLE), according to a study published in Rheumatology.1
Approximately 50% to 80% of patients with SLE are prescribed glucocorticoids indefinitely, even though their long-term side effects are well known.
“The vast majority of physicians (>90%) were willing to reduce or withdraw prednisone before immunosuppressives and antimalarials in cases of prolonged clinical inactivity,” stated investigators at the University of Toronto Lupus Clinic (UTLC).
Patients from UTLC who achieved 2 years of remission were included in this study. Participants were split into either the maintenance group, in which they maintained a low dose (5 mg/day) of prednisone, or the withdrawal group, who followed a tapering schedule over the course of 2 years. Of the 270 eligible patients, with 156 participants placed in the maintenance group and 114 placed in the tapering cohort. Remission was based on the SLE Disease Activity Index 2000 (SLEDAI-2K) of 0. After the initial 2 years, patients had follow-up visits for the following 2 years. Investigators analyzed clinical flares as well as any damage that occurred.
In the withdrawal cohort, their medication was reduced according to this method:
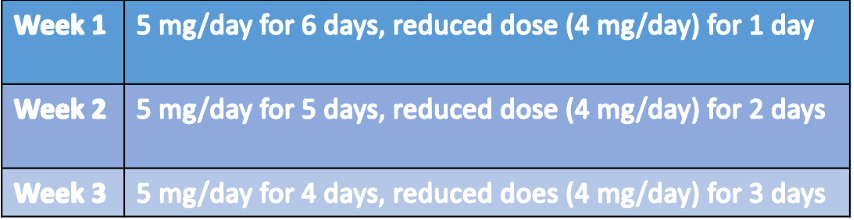
With this approach, participants were able to reach the first reduced dose by Week 7. They then remained on that dose until their next clinic visit, where they were instructed to continue tapering their glucocorticoids. This method ensured patients would ultimately be able to discontinue medication by 9 to 18 months, depending on withdrawal symptoms, personal preferences, and frequency of appointments.
Flares were defined as any increase in SLEDAI-2K (excluding serology), an increase in SLEDAI-2K accompanied by an increase in glucocorticosteroids, antimalarials, or immunosuppressives, or an increase in 4 or more in SLEDAI-2K. Outcomes were analyzed at 12 and 24 months. Damage was assessed at 24 months, based on the Systemic Lupus International Collaborating Clinics Damage Index (SDI).
Patients with flares or increased dosages during the 2-year period were excluded, however, they were studied separately and compared to the maintenance group in order to assess the “success rate” of the tapering approach.
A propensity score-matching (PSM) analysis was performed to account for independent variables such as sex, age, Black race, disease duration, SLEDAI-2K, SDI at index date, and duration of clinical remission prior to the index date. Using this method, 204 patients (102 in each group) were matched.
When compared with the maintenance group, the withdrawal group had lower flare rate at both 12 (17.6%, 29.4%; P = 0.023) and 24 (33.3%, 50%; P = 0.01) months. Increases in SLEDAI-2K or an increase of 4 or more was seen more often in the maintenance group when compared with the tapering cohort at 24 months (51 versus 34, respectively), although half of these flares were considered mild. Symptoms of flare included skin involvement, hematologic involvement, mild arthritis, and mild vasculitic lesions.
Moderate to severe flares were not statistically different between both groups at 12 months, but at 24 months the withdrawal group had far fewer flares (14.7% versus 27.5%, respectively). Additionally, damage was less frequent in the withdrawal cohort when compared with the maintenance group (6.9% versus 17.6%, respectively).
Observational studies are inherently limited and the choice to maintain or taper prednisone was up to the patient’s physician. As the withdrawal rate was based on multiple variables, it was not standardized for all patients. The PSM attempted to mitigate these factors to account for covariates and potentially impacted outcomes, which reduced baseline bias.
“Gradual withdrawal of prednisone seems safer than abrupt discontinuation in patients with clinically quiescent SLE and could be attempted because the vast majority of these patients will not develop a moderate to severe flare within 24 months,” concluded investigators. “A randomized controlled trial could confirm whether gradual glucocorticoid tapering is preferable to abrupt withdrawal in such patients.”
Reference:
Tselios K, Gladman DD, Su J, Urowitz MB. Gradual Glucocorticosteroid Withdrawal Is Safe in Clinically Quiescent Systemic Lupus Erythematosus [published online ahead of print, 2021 Jul 10]. ACR Open Rheumatol. 2021;10.1002/acr2.11267. doi:10.1002/acr2.11267




