Article
Guselkumab Significantly Improves Fatigue in Patients With Psoriatic Arthritis
Author(s):
Guselkumab, an interleukin-23p19-subunit inhibitor, led to clinically significant and sustained improvements in fatigue through 1 year in patients with active psoriatic arthritis.
Guselkumab led to significant and sustained improvements in fatigue through 1 year in patients with active psoriatic arthritis (PsA), according to a study published in Arthritis and Research Therapy.1 Additionally, superior Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scores were independent of effects on other outcomes.
Fatigue is associated closely with chronic inflammatory diseases, and can impact disease activity, pain, sleep, depression, physical and mental work, and health-related quality of life (HRQoL). Guselkumab, an interleukin-23p19-subunit inhibitor, has been clinically proven to treat psoriatic arthritis, but was not previously focused on this symptom.
“The mechanism underlying fatigue is complex and undefined, and it can involve physiological, psychological, and social aspects,” stated investigators. “Signs and symptoms of PsA are effectively treated with biologics that block specific cytokines and reduce inflammation. Improvement of fatigue has also been evaluated in biologic clinical trials, with variable measurement tools and results.”
In the phase 3 DISCOVER-1 and DISCOVER-2 trials, patients with PsA were given either subcutaneous injections of guselkumab 100 mb ever 4 weeks (Q4W, N = 373), guselkumab 100 mg at week 0, 4 and then every 8 weeks (Q8W, N = 375), or placebo (N = 372) through week 24 before switching to Q4W. The DISCOVER-1 trail continued through week 48 and DISCOVER-2 through week 100. All patients enrolled did not respond to or were intolerant of non-biologic disease-modifying antirheumatic drugs (DMARDs), non-steroidal anti-inflammatory drugs, and/or apremilast. Patients in DISCOVER-2 were biologic-naïve. Baseline characteristics and FACIT-Fatigue scores were consistent across the treatment groups and patients exhibited substantial disease burden.
Fatigue was measured using the FACIT-Fatigue instrument, a 13-item patient-reported outcome (PRO) tool that determined the association of fatigue with impact on daily activities and physical function. Scores ranged from 0-52, with higher scores signifying less fatigue.
Mediation analysis aimed to adjust for for improvements that may be indirect of fatigue and instead indicating positive progression in other outcomes, such as the American College of Rheumatology criteria (ACR20), minimal disease activity (MDA), or C-reactive protein (CRP). This was determined post hoc.
According to FACIT-Fatigue scores, patients with PsA had higher levels of baseline fatigue when compared with the general population (29.1-31.4, 43.6, respectively). Patients in the Q4W and Q8W demonstrated significant improvements at week 24 in both DISCOVER-1 and DISCOVER-2 trials (ranging from 5.6 [54%] and 7.6 [63%]) when compared with the placebo group (2.2-3.6 [35-46%]). Additionally, these results were sustained through week 52, with mean changes reported as 6.7-6.9 in DISCOVER-1 and 7.2-8.4 in DISCOVER-2. More patients in both Q4W and Q8W achieved ≥2 to ≥12-point increases when compared with the placebo group by week 24. By week 52, patients in the placebo group who switched to Q4W at week 24 had comparable improvements with those who had originally received guselkumab.
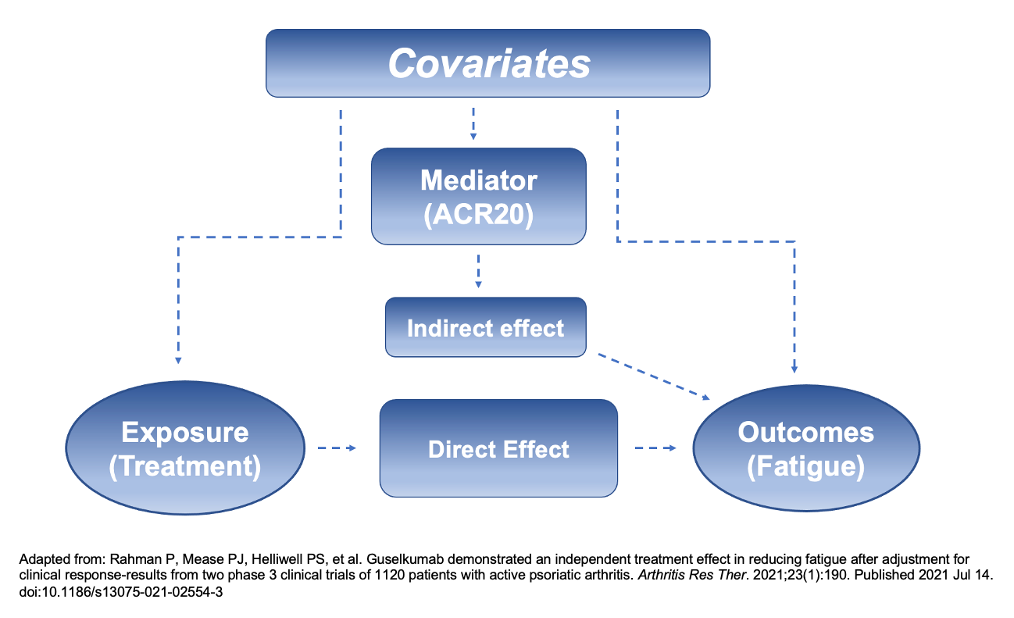
After adjusting for ACR20, MDA, and CRP, analyses indicated much of the improvements on fatigue were a direct effect of receiving guselkumab. In the Q4W cohort, 69% and 70% (DISCOVER-1 and DISCOVER-2, respectively) of the drug’s impact on the FACIT-Fatigue scores was independent of ACR20 and 12% and 36% of the effect was independent of other factors in the Q8W group.
The results of this analysis cannot be compared to other biologic therapies, in which FACIT-Fatigue scores have also been observed, because of differences in assessment tools, study populations, and study designs. Additional research may help differentiate guselkumab from other biologics. Investigators caution interpreting the mediation analyses indicating the drug’s effect on fatigue independent of ACR achievement, MDA response, or CRP due to the complex nature of the symptom. It should be noted that FACIT-Fatigue scores also improved in the placebo group, especially in the DISCOVER-2 trial. Cumulative distribution curves, however, demonstrated that patients receiving guselkumab fared far better.
“Taken together, results of these analyses indicate a strong impact of guselkumab on the fatigue of PsA when assessed via the FACIT-Fatigue instrument,” concluded investigators. “Although further research is needed to more fully characterize the mechanism by which guselkumab improves patient fatigue, our findings may further inform treatment decisions, additional research on this topic, and future consensus deliberations surrounding PsA core set assessments.”
Reference:
Rahman P, Mease PJ, Helliwell PS, et al. Guselkumab demonstrated an independent treatment effect in reducing fatigue after adjustment for clinical response-results from two phase 3 clinical trials of 1120 patients with active psoriatic arthritis. Arthritis Res Ther. 2021;23(1):190. Published 2021 Jul 14. doi:10.1186/s13075-021-02554-3




