Article
Identifying pleomorphic hyalinizing angiectatic tumor: A case report
A pleomorphic hyalinizing angiectatic tumor (PHAT) is a rare soft tissue lesion that mainly affects superficial soft tissue in the lower extremities. PHAT is classified as a borderline/intermediate-grade soft tissue tumor because of the substantial risk of local recurrence.
ABSTRACT: A pleomorphic hyalinizing angiectatic tumor (PHAT) mainly affects superficial soft tissue in the lower extremities. PHAT is classified as a borderline/intermediate-grade soft tissue tumor because of the substantial risk of local recurrence. The diagnosis is based on histological, clinical, immunochemical, and radiological findings. We offer a case report of a middle-aged woman who presented with a painful slow-growing subcutaneous soft tissue mass in her left lateral thigh. An MRI scan showed a tumor mass located within the tensor fascia lata and iliotibial tract. The overall features were suggestive of a PHAT. This lesion often is CD34-positive and almost always is S100-negative; these features are used to differentiate PHAT from other conditions. (J Musculoskel Med. 2009;26:386-388)
A pleomorphic hyalinizing angiectatic tumor (PHAT) is a rare soft tissue lesion that mainly affects superficial soft tissue in the lower extremities. PHAT is classified as a borderline/intermediate-grade soft tissue tumor because of the substantial risk of local recurrence.
The diagnosis of PHAT, which is difficult, mainly is based on histological, clinical, immunochemical, and radiological findings. Histologically, PHAT is characterized by heavily hyalinized vessels; spindled and pleomorphic stromal cells, with intranuclear inclusions; and a variable inflammatory component. There are fewer than 20 reports in the literature about the histological findings1; no paper we know of discusses the radiological findings.
In this article, we offer a case report of a middle-aged woman who presented with a painful slow-growing subcutaneous soft tissue mass in her left lateral thigh. The diagnosis, a soft tissue malignancy (eg, sarcoma), subsequently was confirmed pathologically to be PHAT. We review the clinical, pathological, and radiological features of PHAT. We also discuss the role of imaging and the differential diagnosis of this rare disease entity.
Case report
The patient, a 57-year-old woman with a history of good health, presented to our institution with a 2-year history of a soft tissue mass in her left lateral thigh. The mass was slowly increasing in size, and the patient's pain was thought to be progressing.
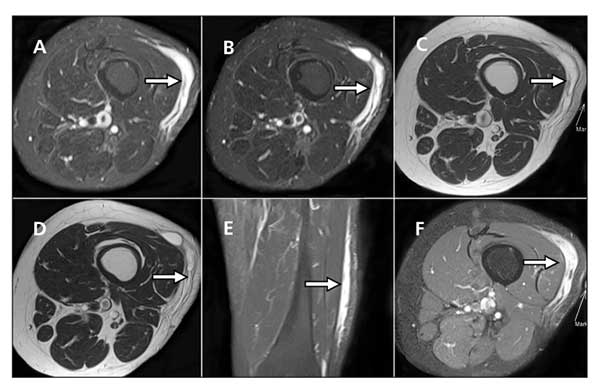
Figure 1 – In a patient with pleomorphic hyalinizing angiectatic tumor, MRI shows a tumor mass (arrows) located in the left lateral thigh superficial soft tissue layer and within the tensor fascia lata and iliotibial tract (A and B, T2 axial views; C and D, T1 axial views; E and F, postcontrast coronal and axial views). The underlying muscles and bony structures are unremarkable. The mass exhibits T1 hypointensity and T2 hyperintensity and heterogeneous contrast enhancement.
An MRI scan showed a 14 × 7 × 1-cm tumor mass located in the patient's left lateral thigh superficial soft tissue layer within the tensor fascia lata and iliotibial tract (Figure 1). The mass was well defined without extension into the underlying muscles and bony structures. It exhibited T1 hypointensity and T2 hyperintensity and heterogeneous contrast enhancement. The overlying subcutaneous layer was mildly edematous.
In view of the interval growth and enhancing MRI features of the mass, it was suggested to be a soft tissue sarcoma. Wide local excision was performed. Microscopically, the tumor mass was a myxoid spindle cell neoplasm with nuclear pleomorphism and hyperchromatism. The tumor mass consisted of lobules and fascicles of spindle cells; it had few mitotic figures. In some areas, fibrinoid thin-walled ectatic vessels were noted. Together with the adjacent adipose tissue infiltration, the overall features were suggestive of a PHAT.
Clinical progress
The pathological result showed positive resection margin, and the follow-up positron emission tomography scan showed a fluid collection with foci of increased fluorodeoxyglucose uptakes in the
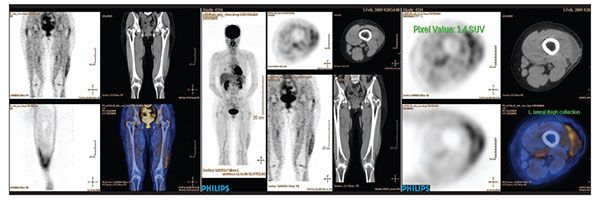
operative bed (Figure 2). Re-excision subsequently was offered to the patient.
Figure 2 – Follow-up positron emission tomography–CT scanning in the patient with pleomorphic hyalinizing angiectatic tumor shows a fluid collection with foci of increased fluorodeoxyglucose uptakes in the operative site. The features are suggestive of residual disease in the presence of postoperative seroma.
Discussion
PHAT is a rare disease entity first described by Smith and associates2 in 1996. Clinically, PHAT is characterized by a slow-growing tumor mass in the superficial/subcutaneous layer of the lower e×tremities. It affects persons in various age-groups (range, 10 to 83 years). Women are affected more frequently than men (ratio, 23:18).3
The World Health Organization categorized PHAT as a benign or low-grade soft tissue tumor. However, some authors think that it is a borderline/intermediate-grade soft tissue malignancy because of its 30% to 50% local recurrence rate.4,5 No metastatic disease resulting from PHAT has been reported in the literature.
The scarcity of mitotic figures in PHAT implies low-grade proliferation. PHAT may be a recurring or residual disease even after wide local excision because it has infiltrative border. Immunochemical staining may be used to help the diagnosis. PHAT often is CD34-positive (80% of cases) and almost always is S100-negative3; these features are used to differentiate PHAT from other conditions.
PHAT affects mostly middle-aged women. Although its radiological features are nonspecific-with a T1 hypointense signal and T2 hyperintense signal and heterogeneous contrast enhancement-its location is characteristic (usually in the superficial or subcutaneous layer). MRI is the imaging modality of choice because of its superior contrast resolution. The roles of imaging in PHAT are localizing the lesion, disease staging, and treatment monitoring.
The differential diagnosis for PHAT includes malignant fibrous histiocytoma (MFH) and neurilemoma. MFH, first described by O'Brien and Stout6 in 1964, is the most common soft tissue sarcoma in adults; it is predominant in men.7 MFH usually has a shorter clinical course than PHAT but is more aggressive.
The location of MFH is a characteristic feature that differentiates it from PHAT. MFH often is deep-seated within the muscular compartment, with invasion into the neurovascular structures. Underlying bony destruction is common.
The angiomatoid subtype of MFH, which arises from the superficial or subcutaneous layer, is a rare exception. Hemorrhage is common in this subtype, however, and fluid-fluid levels are seen in both CT and MRI scans. MFH is intermediate intense in T1-weighted images and heterogeneous hyperintense in T2-weighted images. Because metastasis is common in MFH (40% of cases), imaging of the chest, regional lymph nodes, liver, and bones is warranted. In addition, calcification is seen in 5% to 20% of MFH cases7; no calcification has been reported for PHAT. Histologically, MFH may be differentiated from PHAT with the presence of mitotic figures and CD34 negativity.
Neurilemoma, thought to be a benign soft tissue tumor, is a nerve sheath tumor with a true capsule composed of epineurium.8 The mass characteristically is eccentric with respect to the affected nerve. Clinically, neurilemoma is a slow-growing tumor with an "electric shock" sensation on compression. The Tinel test result usually is positive.
Neurilemoma has characteristic radiological features, including the target and fascicular signs, that may be used to differentiate it from PHAT. The target sign is seen in T2-weighted images with hypointense signal in the center and hyperintense signal in the periphery (because of fibrous components in the former surrounded by myxomatous elements in the latter). The appearance of fascicular bundles in a neurogenic tumor constitutes the fascicular sign. Another distinctive radiological feature of neurilemoma would be depiction of the nerve entering and exiting the mass.
Pathologically, neurilemoma is encapsulated and PHAT would have infiltrative borders. Microscopically, neurilemoma demonstrates Antoni A areas (densely cellular, arranged in short bundles or interlacing fascicles) and Antoni B areas (fewer cells, organized with more myxoid component). S100 positivity and CD34 negativity are other features that differentiate neurilemoma from PHAT.
Differentiating PHAT from its mimics is essential because all involve different management plans and prognosis. Because PHAT is a borderline/intermediate-grade soft tissue tumor with a substantial recurrence rate, it usually is managed with wide local excision. Most MFHs are managed with wide en bloc resection after 3 or 4 months of preoperative chemotherapy; simple excision often is offered for neurilemoma because the nerve is separable from the tumor after incision of the epineurium.9
References:
References1. The Doctor's Doctor. P.H.A.T. http://www.thedoctorsdoctor.com/diseases/phat.htm. Accessed July 20, 2009.
2. Smith ME, Fisher C, Weiss SW. Pleomorphic hyalinizing angiectatic tumor of soft parts: a low-grade neoplasm resembling neurilemoma. Am J Surg Pathol. 1996;20:21-29.
3. Folpe AL, Weiss SW. Pleomorphic hyalinizing angiectatic tumor, analysis of 41 cases supporting evolution from a distinctive precursor lesion. Am J Surg Pathol. 2004;28:1417-1425.
4. Stanford School of Medicine. Pleomorphic Hyalinizing Angioectatic Tumor. http://surgpathcriteria.stanford.edu/softmisc/pleomorphic_hyalinizing_angiectatic_tumor/printable.html. Accessed July 20, 2009.
5. Kazakov DV, Pavlovsky M, Mukensnabl P, Michal M. Pleomorphic hyalinizing angioectatic tumor with a sarcomatous component recurring as high grade myxofibrosarcoma. Pathol Int. 2007;57:281-284.
6. O'Brien JE, Stout AP. Malignant fibrous xanthomas. Cancer. 1964;17:1445-1455.
7. Murphey MD, Gross TM, Rosenthal HG. From the archives of the AFIP (Armed Forces Institute of Pathology). Musculoskeletal malignant fibrous histiocytoma: radiologic-pathological correlation. Radiographics. 1994;14:807-828.
8. Beaman FD, Kransdorf MJ, Menke DM. Schwannoma: radiologic-pathologic correlation. Radiographics. 2004;24:1477-1481.
9. Dahnert W. Musculoskeletal neurogenic tumor. In: Dahnert W. Radiology Review Manual. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2003:189-190.




