Article
Intraviteral Ranibizumab Effective Treatment for AMD with Submacular Hemorrhage
Author(s):
83.4% of trial study participants showed either improvement or stabilization in best-corrected visual acuity with ranibizumab.
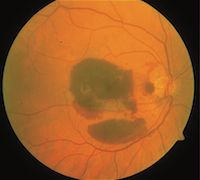
Submacular Hemorrhaging in AMD
A study to evaluate safety and efficacy of anti-vascular endothelial growth factor (anti-VEGF) agent ranibizumab on patients with submacular hemorrhage (SMH) associated with neovascular age-related macular degeneration (wAMD) determined that the treatment was not only safe and effective but was associated with stabilization and improvements in visual acuity.
The study, led by Dimitrios Karagiannis, MD, FEBO, and colleagues with the University of Athens, in Greece, found that 83.4% of trial study participants showed either improvement or stabilization in best-corrected visual acuity [BCVA] after treatment at the 1-year mark. Karagiannis and colleagues determined that the treatment outcomes, however, were affected by the location of the SMH in patients.
The study, designed to provide that missing information on safety and efficacy of intravitreal ranibizumab treatment for SMH due to AMD, included 12 patients (5 male, 7 female), aged 50 years or older (mean age = 80.3±3.6 years) with treatment-naïve SMH secondary to wAMD and SMH choroidal neovascularization >50%.
The authors reported that patients received "3 consecutive monthly intravitreal injections of 0.5 mg/0.05 mL ranibizumab and as needed [during the 12-month study] thereafter if visual deterioration more than 1 Snellen line" during tests of BCVA, or if there was evidence of persistent or recurrent fluid or hemorrhage observed during monthly follow-ups.
Data from the study showed that BCVA at baseline improved by 2 or more Snellen lines and stabilization was improved for 41.7% of patients, and only 3 out of the 12 patients saw a recurrence in SMH. Study data also determined that location of SMH presentation in the foveal area was predictive of better or worse anatomical and visual outcomes after treatment. Patients with SMH surrounding the foveal area (360º) presented worse outcomes post-treatment than those patients with SMH adjacent, but not "encircling," the fovea.
Karagiannis and colleagues report that mean BCVA improved to 0.38 from 0.28 at 12 months and concluded that intravitreal ranibizumab "seems to be safe and effective, either improving or stabilizing VA in patients with SMH due to wet AMD."
The study, although limited in scope and selection of the anti-VEGF agent, suggests that use of intravitreal anti-VEGF therapy for treatment of SMH secondary to wAMD may provide safer more effective treatment for patients than existent therapies for SMH due to AMD.
Karagiannis noted that wAMD can be complicated by SMH, which can also lead to progressive or sudden visual loss in patients. SMH is the result of blood building up between the neurosensory retina and the retinal pigment epithelium (RPE). This accumulation of subretinal blood creates a toxic environment which contributes to retinal damage, which is considered irreversible, although there are a variety of treatment options which attempt to thwart the spread of that damage. A variety of SMH treatments were explored, but the authors asserted that many are associated with serious complications, which create limitations on both effectiveness and safety.
Karagiannis and colleagues pointed to advancements in the use of intravitreal anti-VEGF agents like ranibizumab for the treatment of wAMD, calling those treatments the "gold standard," but mentioned that during anti-VEGF trials, patients with SMH due to AMD were often excluded.
Karagiannis wrote that because of this, the efficacy and safety data for use of ranibizumab in patients with SMH due to AMD has not been established and it led to a lack of information about the "anatomical and functional outcomes" of anti-VEGF treatment based on the location of the SMH.
The study, "Location of submacular hemorrhage as a predictor of visual outcome after intravitreal ranibizumab for age-related macular degeneration,” appeared in Clinical Interventions in Aging.


