Article
Iontophoretic Administration of Dexamethasone for Musculoskeletal Pain
Musculoskeletal pain is common. In one analysis, 33% of adults reported musculoskeletal pain from overuse as a chief complaint. Morbidity resulting from musculoskeletal pain contributes to 29% of lost workdays because of illness and is second only to cardiovascular disease in terms of economic burden
ABSTRACT: Corticosteroids are highly effective in reducing inflammation and present an appropriate pharmacotherapeutic option for many musculoskeletal disorders. However, systemic administration carries the risk of collateral damages. Transdermal administration of corticosteroids via iontophoresis allows for localized delivery, thus bypassing many of the problems with systemic administration. The potential safety benefits are compelling, but efficacy data have been conflicting. Most data suggest that iontophoresis facilitates dexamethasone penetration into human tissues. We reviewed 13 clinical trials that met criteria for muscle soreness, carpal tunnel syndrome, osteoarthritis of the knee, rheumatoid arthritis of the knee, plantar fasciitis, trapeziometacarpal arthritis, Achilles tendon pain, and epicondylitis. (J Musculoskel Med. 2011;28:410-421)
____________________________________________________________________________________
Musculoskeletal pain is common. In one analysis, 33% of adults reported musculoskeletal pain from overuse as a chief complaint. Morbidity resulting from musculoskeletal pain contributes to 29% of lost workdays because of illness and is second only to cardiovascular disease in terms of economic burden.1 Chronic symptoms related to musculoskeletal pain affect not only patients’ physical function but also their mental and emotional well-being.2
Corticosteroids are used widely to benefit patients who experience musculoskeletal pain, as monotherapy or as part of a multifaceted approach. Injection is the most widely used method for delivering corticosteroids to a localized area.
Corticosteroids are highly effective in reducing inflammation and therefore present an appropriate pharmacotherapeutic option for many musculoskeletal disorders. Systemic administration of corticosteroids carries the risk of a variety of collateral damages, especially with repeated use. The systemic adverse effects include glucose dysregulation, psychosis, immunosuppression, osteonecrosis, hypertension, hyperlipidemia, and appearance changes.3 In addition, receiving corticosteroid injections may be painful and traumatic.
Transdermal administration of corticosteroids via iontophoresis allows for localized delivery, thus bypassing many of the unwanted toxicities and nuisances, as well as the invasiveness of an injection. In this article, we provide a concise review of the literature related to iontophoresis of dexamethasone in musculoskeletal conditions.
FIGURE
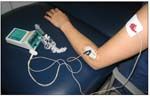
A patient with epicondylitis is receiving dexamethasone via iontophoretic administration. The electrodes are placed strategically to facilitate treatment. (Photograph courtesy of Krupalee Brunetti, DPT.)
BACKGROUND
Iontophoresis of corticosteroids has been used clinically since the 1950s (Figure).4 The mechanism involves active transport within an electric field. The basic principles are based on the physical phenomenon that “like charges repel and opposite charges attract.” Anionic drugs are forced across the skin by using a negatively charged working electrode. Similarly, cationic drugs enter the skin when a positively charged electrode is used. Once the drug permeates the skin, it reaches its target tissue by rapidly diffusing into the local circulation.
The delivery of corticosteroids using the technology of iontophoresis has several advantages over parenteral administration. Transdermal administration with iontophoresis avoids systemic administration and bypasses the hepatic “first pass” metabolism, which ultimately reduces the variation of absorption seen with oral administration. As an alternative to systemic administration, iontophoresis minimizes some of the unwanted consequences related to injections (eg, tendon rupture and skin and fat pad atrophy).
In addition, patients may be more willing to receive local treatments than an invasive injection. Drug delivery is programmed by the amount of voltage of the current applied, allowing the administrator to deliver a very precise dose. The iontophoretic delivery system also may be turned off, leading to, if needed, rapid termination of the medication.
Iontophoretic administration of dexamethasone has been evaluated in a variety of musculoskeletal conditions. Although the potential safety benefits are compelling, efficacy data have been conflicting.
IONTOPHORETIC ADMINISTRATION
For drugs to be viable candidates for iontophoresis, they must have the ability to transfer electrical energy (must be charged). In addition, both drug concentration and drug physiochemical properties must be considered because they influence the extent of transdermal delivery. Data on the effectiveness of iontophoresis in facilitating corticosteroid penetration through the skin are conflicting.
Glass and colleagues5 evaluated penetration of dexamethasone administered with iontophoresis into the tissue of rhesus monkeys. The researchers concluded that dexamethasone readily penetrates the epidermis and reaches the desired joint layers (muscle, synovium, joint capsule, and cartilage) at depths of 17 mm.
In another study, Gurney and associates6 evaluated the penetration of cathodic iontophoretic (40-mA-minute dose) administration of 0.4% dexamethasone in 16 adults undergoing anterior cruciate ligament reconstruction. Before surgery, a slip of the distal semitendinosus tendon was exposed to treatment. The slip was extracted within 4 hours, and skin-fold thickness was measured. The authors concluded that iontophoretic administration of dexamethasone penetrates human connective tissue with skin-fold thickness up to 30 mm. Of note, only 7 of the 16 patients had measurable dexamethasone in the tendon slip.
In another analysis, in vitro cathodic iontophoresis at 4 mA for 10 minutes (followed by 400 minutes of diffusion time) and 0.1 mA for 400 minutes of 0.4% dexamethasone both provided a penetration depth of 12 mm into hydrogel.7 Of note, penetration of drug ions into the hydrogel during the active transport process of iontophoresis did not surpass 2 mm. The analysis provided evidence that after iontophoretic administration, a drug depot is formed in the epidermis and then the drug is passively absorbed. The extent of passive diffusion is influenced by cutaneous vasoconstriction, which is related to the depth of tissue penetration.8 These data suggest that for complete understanding of the mechanism of iontophoretic administration of dexamethasone, a physician should place the focus on the influence of the current and medications on vascular tone.
Contrary to the above findings, iontophoretic administration of dexamethasone into the equine tibiotarsal joint did not yield any measurable drug concentration.9 Similarly, in a separate analysis, drug concentrations in a vein proximal to the iontophoretic treatment area were not measurable.10
In spite of these conflicting findings, most data suggest that iontophoresis facilitates dexamethasone penetration into human tissues. They also suggest that the extent of penetration is related to tissue thickness, treatment time, and local blood flow.
DATA SOURCES AND SELECTION
A medical literature search was conducted in MEDLINE/PubMed (1950 - August 2010), Web of Science (1980 - August 2010), International Pharmaceutical Abstracts (1977 - August 2010), and Google Scholar; the terms “dexamethasone,” “iontophoresis,” and “musculoskeletal disorders” were used to identify relevant original research articles. References from publications identified were reviewed for additional resources. All articles in English identified from the data sources were evaluated. Priority was placed on data derived from humans, especially those obtained from randomized controlled trials. Data from clinical trials that evaluated temporomandibular pain were excluded.
A total of 13 clinical trials that met the inclusion/exclusion criteria were reviewed and evaluated. In those trials, iontophoretic administration of dexamethasone was evaluated in the following musculoskeletal conditions: muscle soreness (n = 1), carpal tunnel syndrome (CTS) (n = 3), osteoarthritis (OA) of the knee (n = 1), rheumatoid arthritis (RA) of the knee (n = 1), plantar fasciitis (n = 3), trapeziometacarpal (TMC) arthritis (n = 1), Achilles tendon pain (n = 1), and epicondylitis (n = 2).
Effectiveness
Several studies have assessed the effectiveness of corticosteroid iontophoresis in a variety of musculoskeletal ailments. Tables 1 - 7 summarize efficacy data from dexamethasone iontophoretic applications in these patient populations.
TABLE 1

Dexamethasone iontophoresis for muscle soreness efficacy dataTABLE 2

Dexamethasone iontophoresis for CTS efficacy dataTABLE 3

Dexamethasone iontophoresis for OA or RA of the knee efficacy dataTABLE 4
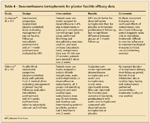
Dexamethasone iontophoresis for plantar fasciitis efficacy data TABLE 5
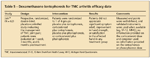
Dexamethasone iontophoresis for TMC arthritis efficacy date TABLE 6

Dexamethasone iontophoresis for Achilles tendon pain efficacy data TABLE 7

Dexamethasone iontophoresis for epicondylitis efficacy data
Of the 3 clinical studies in which iontophoretic administration of dexamethasone in patients with CTS pain was evaluated,11-13 only 1 was a randomized, placebo-controlled, blinded study.13 The authors concluded that iontophoresis of dexamethasone is no better than placebo and thus confers no added clinical benefit. The sample size was small in all of the studies, with a collective total of 65 patients. Overall, the studies do not provide evidence that iontophoretic administration of dexamethasone confers significant benefit in CTS.
The utility of iontophoresis plus dexamethasone in pain related to OA or RA of the knee was evaluated in 2 small studies. In a pilot study of 10 patients, those who received dexamethasone iontophoresis had an improvement in pain at rest and on movement compared with placebo.14 In a separate analysis (N = 50), an improvement in Western Ontario and McMaster University Osteoarthritis Index scores from baseline was seen in patients with a diagnosis of OA of the knee treated with phonophoresis or iontophoresis plus dexamethasone (60% vs 64% of patients, respectively); however, there were no differences noted between groups.15 Although these data are of interest, they need confirmation from large, randomized, placebo-controlled studies.
There have been 3 well-designed, randomized, double-blind, placebo-controlled clinical trials that evaluated the effectiveness of dexamethasone iontophoresis in managing plantar heel pain.16-18 Two studies showed improvement in pain; however, follow-up was short, at 1 month.16,17 In addition, both studies used multiple treatment modalities, making it difficult to rule out possible confounding. Contrary to the findings in these studies, one analysis found that dexamethasone iontophoresis plus exercise is no better than manual physical therapy plus exercise at 1 month and at 6 months.18
These data suggest that iontophoresis may be beneficial in the short-term (acute-phase) management of plantar heel pain.18 However, no recommendation can be made on long-term benefits.
Jain and colleagues19 completed a randomized, placebo-controlled, double-blind study to evaluate dexamethasone iontophoresis for the management of TMC arthritis pain. End points were well defined and based on validated scales, such as the Michigan Hand Questionnaire, 12-Item Short-Form Health Survey, analog pain score, and provocative and strength testing. No benefit was noted in iontophoretic administration of dexamethasone. The authors concluded that transdermal delivery of corticosteroids might not be helpful in managing arthritis pain in both the short and long term.
Neeter and coworkers20 evaluated the effectiveness of dexamethasone iontophoresis in managing Achilles tendon pain in 25 patients. The toe-raise test; dorsal flexion; plantar flexion; and patient reports of pain during and after activities, walking, and walking up and down stairs were evaluated at baseline and at 2 weeks, 6 weeks, 3 months, and 6 months. No differences were noted between the study and control groups in the toe-raise test or range of motion tests at any of the prespecified end points. There were significant improvements in patient-reported pain during physical activity in the study group; however, the improvement was significant only after physical activity at the 6-month follow-up.
Only 2 end points were significantly improved from baseline and compared with the control group, pain during walking at 6 months and pain while walking up and down stairs at 6 months. Significantly fewer patients in the dexamethasone group reported pain during physical activity at the 1-year follow-up (2/14 vs 6/10) or after physical activity (2/14 vs 6/10) compared with the control group. The authors concluded that dexamethasone iontophoresis has a role in managing acute Achilles tendon pain.
Two studies evaluated the effectiveness of dexamethasone iontophoresis in managing epicondylitis, both with a randomized, placebo-controlled, double-blind design. Nirschl and associates21 conducted the largest study (N = 199) to date that evaluated dexamethasone iontophoresis in a musculoskeletal condition. A significant improvement in Visual Analog Scale pain rating was appreciated in the dexamethasone iontophoresis group compared with the placebo group (24 mm vs 19 mm) at 1 month. These data suggest that dexamethasone iontophoresis has at least a short-term benefit in epicondylitis.
In a separate study, Runeson and Haker22 were not able to reproduce the benefits of dexamethasone iontophoresis. There was no significant difference seen in any of the measured end points between the treatment and placebo groups. Of note, this study enrolled fewer patients (N = 64) and probably lacked sufficient power to provide meaningful results.
Although several studies have evaluated dexamethasone iontophoresis, the findings are limited by several drawbacks. All of the clinical trials to date have enrolled somewhat small samples (with the exception of Nirschl and associates21). In addition, long-term outcomes were not evaluated. The outcome measures varied among the studies, and not all of them represent clinically relevant end points. Also, the studies varied in design and condition, making it difficult to extrapolate the results to the general population; the interventions were not consistent from study to study; and the iontophoresis and dexamethasone doses and concomitant treatment modalities varied.
Future research should include larger sample sizes, and the intervention should be consistent with what is used in current practice. Until additional data suggest otherwise, iontophoretic administration of dexamethasone cannot be recommended as an effective treatment modality in musculoskeletal disorders as monotherapy.
Dexamethasone iontophoresis may be more appropriate as adjunctive therapy or part of a rehabilitation plan. Limited data suggest benefit, and the majority of those that do suggest benefit do so only in the acute phase of the malady. If the treatment modality is used, the duration of use should be limited.
Safety
Iontophoresis generally is well tolerated; the most common adverse effects include warmth, itching, and tingling at the application site. Erythema and urticaria also have been reported beneath electrodes, but these effects are transient.
Iontophoretic burns may occur and usually are the result of faulty electrodes, inappropriate placement of electrodes, inappropriate dosage, or electrochemical burns. The reported incidence of burns ranges from 1 in 10,000 to 1 in 20,000 treatments.23 Adequate physician training and well-defined protocols minimize the potential of harm resulting from iontophoresis administration. Physicians also should be aware of the contraindications to iontophoresis, which include diabetes mellitus (relative contraindication), use of a pacemaker, pregnancy, wounds present in the area of application, and any hypersensitivities to the drug applied or electrical current.24
CONCLUSION
Transdermal delivery of dexamethasone using iontophoresis is an intriguing treatment option that offers the patient dexamethasone treatment but circumvents the toxicities associated with injectable dexamethasone. Although iontophoretic administration of dexamethasone is used frequently in clinical practice, efficacy data are conflicting.
Larger-scale clinical trials of longer duration are needed to elucidate the role of dexamethasone iontophoresis in managing common musculoskeletal disorders. However, its inclusion as a component in a multifaceted treatment plan may provide short-term benefit with little risk in select musculoskeletal conditions.
Problems/comments about this article? Pleasesend feedback.
References:
References
1. Global Year Against Musculoskeletal Pain: October 2009 â October 2010. Musculoskeletal Pain Fact Sheets. http://www.iasp-pain.org/AM/AMTemplate.cfm?Section=Home&CONTENTID=9287&TEMPLATE=/CM/ContentDisplay.cfm&SECTION=Home. International Association for the Study of Pain. Accessed October 5, 2011.
2. Foster NE, Thomas E, Bishop A, et al. Distinctiveness of psychological obstacles to recovery in low back pain patients in primary care. Pain. 2010;148:398-406.
3. Citterio F. Steroid side effects and their impact on transplantation outcome. Transplantation. 2001;72(12 suppl):S75-S80.
4. Zempsky WT, Ashburn MA. Iontophoresis: noninvasive drug delivery. Am J Anesthesiol. 1998;25:158-162.
5. Glass JM, Stephen RL, Jacobson SC. The quantity and distribution of radiolabeled dexamethasone delivered to tissue by iontophoresis. Int J Dermatol. 1980;19:519-525.
6. Gurney B, Wascher D, Eaton L, et al. The effect of skin thickness and time in the absorption of dexamethasone in human tendons using iontophoresis. J Orthop Sports Phys Ther. 2008;38:238-245.
7. Anderson CR, Morris RL, Boeh SD, et al. Effects of iontophoresis current magnitude and duration on dexamethasone deposition and localized drug retention. Phys Ther. 2003;83:161-170.
8. Singh P, Roberts MS. Effects of vasoconstriction on dermal pharmacokinetics and local tissue distribution of compounds. J Pharm Sci. 1994;83:783-791.
9. Blackford J, Doherty TJ, Ferslew KE, Panus PC. Iontophoresis of dexamethasone-phosphate into the equine tibiotarsal joint. J Vet Pharmacol Ther. 2000;23:229-236.
10. Smutok MA, Mayo MF, Gabaree CL, et al. Failure to detect dexamethasone phosphate in the local venous blood postcathodic iontophoresis in humans. J Orthop Sports Phys Ther. 2002;32:461-468.
11. Banta CA. A prospective, nonrandomized study of iontophoresis, wrist splinting, and antiinflammatory medication in the treatment of early-mild carpal tunnel syndrome. J Occup Med. 1994;36:166-168.
12. Gökoglu F, Fndkoglu G, Yorgancoglu ZR, et al. Evaluation of iontophoresis and local corticosteroid injection in the treatment of carpal tunnel syndrome. Am J Phys Med Rehabil. 2005;84:92-96.
13. Amirjani N, Ashworth NL, Watt MJ, et al. Corticosteroid iontophoresis to treat carpal tunnel syndrome: a double-blind randomized controlled trial. Muscle Nerve. 2009;39:627-633.
14. Li LC, Scudds RA, Heck CS, Harth M. The efficacy of dexamethasone iontophoresis for the treatment of rheumatoid arthritic knees: a pilot study. Arthritis Care Res. 1996;9:126-132.
15. Akinbo SR, Aiyejusunle CB, Akinyemi OA, et al. Comparison of the therapeutic efficacy of phonophoresis and iontophoresis using dexamethasone sodium phosphate in the management of patients with knee osteoarthritis. Niger Postgrad Med J. 2007;14:190-194.
16. Gudeman SD, Eisele SA, Heidt RS Jr, et al. Treatment of plantar fasciitis by iontophoresis of 0.4 % dexamethasone: a randomized, double-blind, placebo-controlled study. Am J Sports Med. 1997;25:312-316.
17. Osborne HR, Allison GT. Treatment of plantar fasciitis by LowDye taping and iontophoresis: short term results of a double blinded, randomised, placebo controlled clinical trial of dexamethasone and acetic acid. Br J Sports Med. 2006;40:545-549.
18. Cleland JA, Abbott JH, Kidd MO, et al. Manual physical therapy and exercise versus electrophysical agents and exercise in the management of plantar heel pain: a multicenter randomized clinical trial. J Orthop Sports Phys Ther. 2009;39:573-585.
19. Jain R, Jain E, Dass AG, et al. Evaluation of transdermal steroids for trapeziometacarpal arthritis. J Hand Surg. 2010;35A:921-927.
20. Neeter C, Thomeé R, Silbernagel KG, et al. Iontophoresis with or without dexamethazone in the treatment of acute Achilles tendon pain. Scand J Med Sci Sports. 2003;13:376-382.
21. Nirschl RP, Rodin DM, Ochiai DH, Maartmann-Moe C; DEX-AHE-01-99 Study Group. Iontophoretic administration of dexamethasone sodium phosphate for acute epicondylitis: a randomized, double-blinded, placebo-controlled study. Am J Sports Med. 2003;31:189-195.
22. Runeson L, Haker E. Iontophoresis with cortisone in the treatment of lateral epicondylalgia (tennis elbow)-a double-blind study. Scand J Med Sci Sports. 2002;12:136-142.
23. Rattenbury JM, Worthy E. Is the sweat test safe? Some instances of burns received during pilocarpine iontophoresis. Ann Clin Biochem. 1996;33(pt 5):456-458.
24. Harris PR. Iontophoresis: clinical research in musculoskeletal inflammatory conditions. J Orthop Sports Phys Ther. 1982;4:109-112.
25. Hasson SM, Wible CL, Reich M, et al. Dexamethasone iontophoresis: effect on delayed muscle soreness and muscle function. Can J Sport Sci. 1992;17:8-13.




