Article
JAK Inhibitors may Serve as Additional Option to Treat Psoriasis, Psoriatic Arthritis
Author(s):
“This class of medication provides an alternative therapeutic option for those that have an inadequate response to conventional DMARDs or biologic therapy.”
Janus Kinase (JAK) inhibitors, a type of small molecule targeted synthetic disease-modified antirheumatic drug (DMARD), show potential for safety treating patients with moderate-to-severe psoriasis and psoriatic arthritis, according to a systemic review of primary research literature published in BMC Rheumatology examining efficacy and safety concerns.1
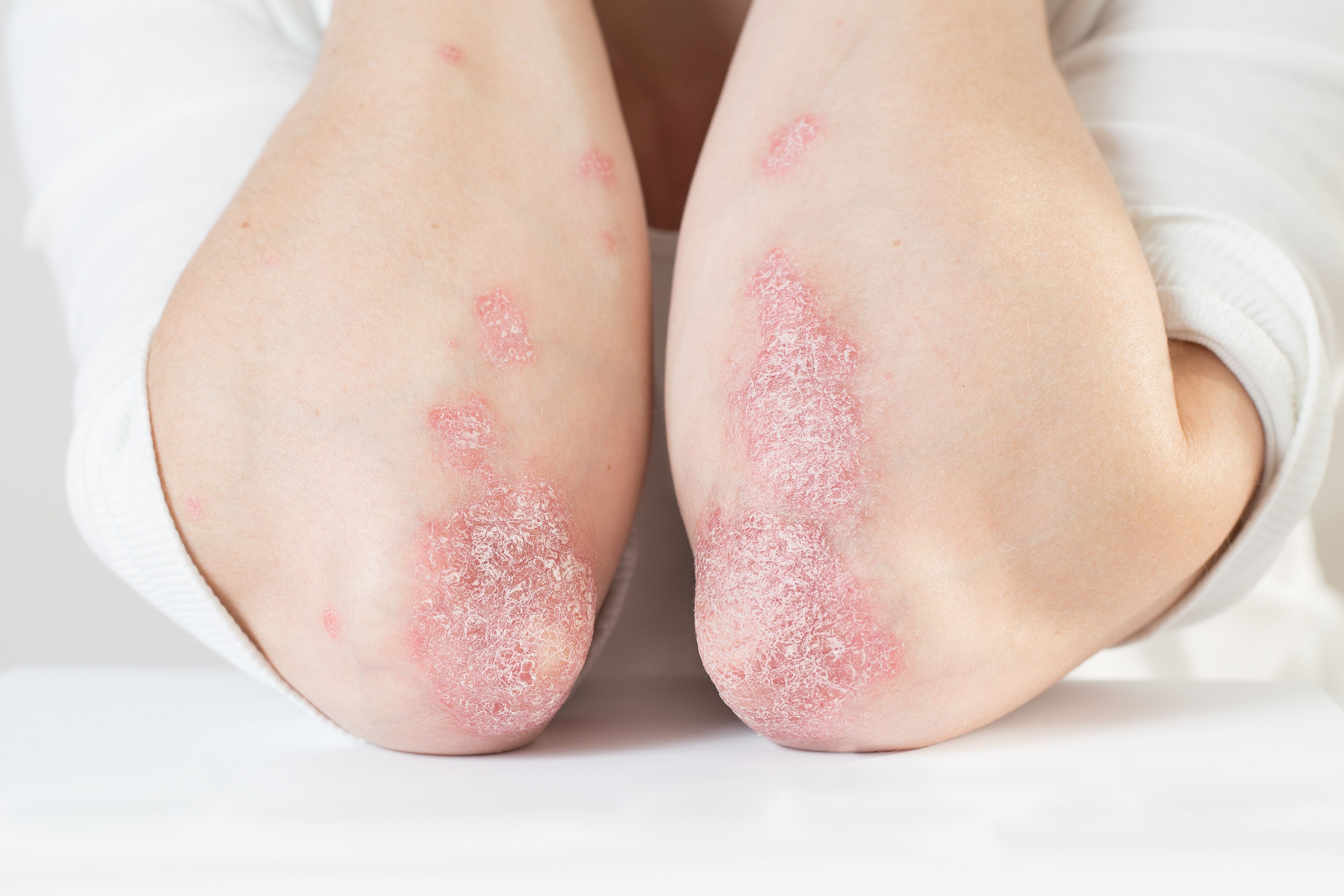
“Inhibition of the JAK/signal transducer and activator of transcription (STAT) pathway prevents the upregulation of pro-inflammatory genes involved in articular and extraarticular inflammation, by modulating cytokine signaling that are integral to lymphocyte activation, proliferation, and function,” investigators stated. “This class of medication provides an alternative therapeutic option for those that have an inadequate response to conventional DMARDs or biologic therapy. Furthermore, in rheumatoid arthritis, it has been shown to have relatively lower infection rates compared to biologic DMARDs.”
The databases MEDLINE, Cochrane, and EMBASE were used to find randomized controlled trials (RCTs) and observational studies that compared JAK inhibitors with placebo in adult patients with moderate-to-severe plaque psoriasis or psoriatic arthritis (PsA). The primary endpoints included a 75% improvement in the Psoriasis Area and Severity Index (PASI75), a standard outcome measure for psoriasis, as well as a 20% improvement in the American College of Rheumatology composite score (ACR20), a 5-point scale that measures severity. A secondary endpoint was the proportion of patients who achieved either a “0” or “1” score on the static Physician Global Assessment scale. Odds ratio analyzed the proportion of patients who were able to reach these targets in the max dose cohort compared with the placebo cohort.
Ultimately, 15 RCTs, comprised of 6757 patients, were included in the analysis, with 7 comparing oral tofacitinib with placebo. All studies focusing on tofacitinib reported a significant improvement in the proportion of patients that reached PASI75 (odds ratio [OR] 14.35 (95%CI 7.65, 26.90). When calculating the PASI75 among non-tofacitinib JAK inhibiting drugs compared with placebo, OR was 6.42 (95%CI 4.89, 8.43). ACR20 among all JAK inhibitors versus placebo was OR 5.87 (95%CI 4.39, 7.85). No significant differences in the prevalence of serious adverse events (AEs) were reported between intervention and control groups.
The lack of observational studies limits the data. Additionally, data was limited to a maximum of 24 weeks for placebo-controlled treatment and 52 weeks of follow-up in most cases, thus limiting long-term safety and efficacy outcomes. However, only including phase 2 and phase 3 RCTs strengthens the study. The high statistical heterogeneity in some of the analyses may be attributed to the large range of sample sizes throughout the studies, however; no significant clinical heterogeneity was identified.
“More research will need to be done to directly compare JAK inhibitors to each other and to other therapies with different mechanisms of action to determine their optimal role in treating psoriatic disease and its various manifestations,” investigators concluded. “Data will be needed on whether JAK inhibitors can be used as monotherapy or whether they need background conventional DMARDs to be effective. The post marketing information on these medications is limited and more data will be needed to ensure the safety and efficacy of JAK inhibitors in the long term. Further research will also be required on other patient subgroups, including older patients and those with comorbid immunocompromising conditions such as diabetes and chronic kidney disease. This information will be important to estimate real word effects and impact of these therapies.”
Reference:
Sarabia S, Ranjith B, Koppikar S, Wijeratne DT. Efficacy and safety of JAK inhibitors in the treatment of psoriasis and psoriatic arthritis: a systematic review and meta-analysis. BMC Rheumatol. 2022;6(1):71. Published 2022 Sep 27. doi:10.1186/s41927-022-00287-7




