Article
JAK Treatment Increases Risk for Adverse COVID-19 Outcomes
Author(s):
The risk of mortality was significantly higher in patients with RA treated with JAKs who had not received the COVID-19 vaccine.
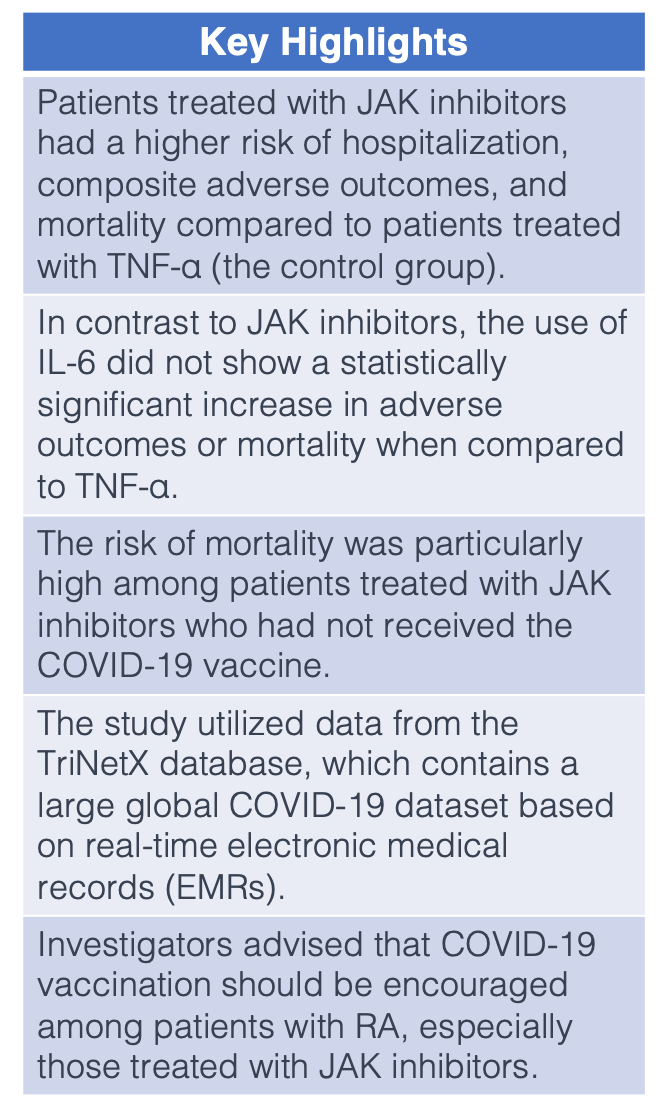
Patients with rheumatoid arthritis (RA) treated with Janus kinase inhibitors (JAK) were at a significant risk for hospitalization, composite adverse outcomes, and mortality, according to a study published in Rheumatic and Musculoskeletal Diseases.1 This risk was particularly high among patients who did not receive the COVID-19 vaccination.
Disease-modifying antirheumatic drugs (DMARDs) have played a critical role in the treatment of RA and other rheumatic diseases over the past 2 decades. However, in recent years, COVID-19 infection has been linked to an exaggerated immune response driven by these medications and cytokine storms. In fact, immune activation patterns in patients with RA seem to be comparable to patients with COVID-19.2
“A previous international registry study of the COVID-19 Global Rheumatology Alliance (C19-GRA) analyzed people with RA using biologic or targeted DMARDs (b/tsDMARDs) at the time of COVID-19 onset and investigated COVID-19 outcomes from March 2020 to April 2021,” explained Shiow-Ing Wang, PhD, Center for Health Data Science, Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan, and colleagues. “Compared with the use of TNF, people with RA using rituximab or JAK were more likely to experience poor COVID-19 outcomes.”
To better understand the role of b/tsDMARD exposure in COVID-19 outcomes among patients with RA, data was extracted from the TriNetX database (a US Collaborative Network) between January 2018 and December 2022. The b/tsDMARDs included JAKs and interleukin-6 inhibitors (IL-6), while tumor necrosis factor-alpha inhibitors (TNF-α) were used as a control group.
COVID-19 outcomes of interest were the incidence of infection and adverse outcomes, which included critical care services, mechanical ventilation, hospitalization, and mortality. The hazard ratio (HR) and 95% confidence interval (CI) were determined using propensity score matched (PSM) patients with different b/tsDMARDs. Information on demographics, lifestyle variables, comorbidities, procedures, and medications were included.
After PSM, 2 groups emerged: JAK (n = 2676) vs TNF (n = 2676) and IL-6 (n = 967) vs TNF (n = 967). No statistical significance was observed regarding the incidence of COVID-19 in patients receiving JAK treatment when compared with those receiving TNF (HR: 1.058, 95% CI: .895 to 1.250). Similar results were seen in IL-6 group compared with the TNF group (HR: 1.028, 95% CI: .779 to 1.358).
However, patients with RA treated with a JAK reported a significantly higher risk for hospitalization (HR: 1.194, 95% CI: 1.003 to 1.423), composite adverse outcomes (HR: 1.242, 95% CI: 1.051 to 1.468), and mortality (HR: 1.440, 95% CI: 1.049 to 1.976) when compared with the TNF group. The risk of mortality was significantly higher in the JAK cohort who had not received the COVID-19 vaccine (HR: 1.511, 95% CI: 1.077 to 2.121).
These risks were not observed in patients receiving IL-6 compared with TNF, including adverse outcomes (HR: 1.209, 95% CI: .924 to 1.581) and mortality (HR: .835, 95% CI: .517 to 1.348).
Investigators noted the TriNetX database as a strength of the study, which currently contains the largest global COVID-19 dataset and uses real-time electronic medical records (EMRs). However, although the study population was racially diverse, over 80% of participants were American, which hindered generalizability to other parts of the world. Additionally, EMR studies are subject to possible misclassification bias and residual confounding.
“COVID-19 vaccination should be encouraged in these target cohorts,” investigators concluded. “When using JAK inhibitors for patients with RA, clinicians should be vigilant about these adverse outcomes to prevent their occurrence or detect them early for early intervention.”
References
- Tsai JJ, Liu LT, Chen CH, Chen LJ, Wang SI, Wei JC. COVID-19 outcomes in patients with rheumatoid arthritis with biologic or targeted synthetic DMARDs. RMD Open. 2023;9(3):e003038. doi:10.1136/rmdopen-2023-003038
- Dewanjee S, Kandimalla R, Kalra RS, et al. COVID-19 and rheumatoid arthritis crosstalk: emerging association, therapeutic options and challenges. Cells 2021;10:3291. doi:10.3390/cells10123291





