Article
Keeping acute rheumatic fever in the differential
Acute rheumatic fever (ARF) was a major cause of morbidity and mortality in past decades, but with the passage of time and medical advances, it faded from the forefront of clinical medicine and, in turn, from physicians' minds. However, a resurgence of ARF has been reported in the United States and the condition remains a significant health concern in the developing world.
ABSTRACT: Acute rheumatic fever, a major cause of morbidity and mortality in the past, remains a significant health concern, especially in the developing world. Rheumatic heart disease is a major sequela and the primary cause of acquired valvular disease worldwide. Arthritis is the most common symptom and often the first to appear. Cardiac involvement may include valvulitis, myocarditis, and pericarditis. Chorea occurs in some patients. Many physicians use the 1992 update of the Jones criteria to make the diagnosis. Treatment should be tailored to the patient's unique set of symptoms. Current prevention efforts are focused on primary and secondary prophylaxis. (J Musculoskel Med. 2009;26:377-385)
Acute rheumatic fever (ARF) was a major cause of morbidity and mortality in past decades, but with the passage of time and medical advances, it faded from the forefront of clinical medicine and, in turn, from physicians' minds. However, a resurgence of ARF has been reported in the United States and the condition remains a significant health concern in the developing world.
Although ARF can be somewhat brief and self-limited, one of its major sequelae, rheumatic heart disease (RHD), has severe and lasting consequences and remains the primary cause of acquired valvular disease.1 As the world becomes more and more interconnected, physicians would do well to maintain ARF as part of their differential diagnosis to allow for earlier diagnosis, management, and prevention of RHD.
In this article, we review the causes, genetics, epidemiology, clinical features, and diagnosis of ARF. Then we discuss approaches to treatment and prevention.
ETIOLOGY
The causes of ARF are complex and not entirely understood, but the condition is known to be an autoimmune reaction directed against the tissues of the heart and brain after group A streptococcal (GAS) pharyngitis. Multiple studies have shown that cross-reactive antibodies formed mainly in response to the streptococcal M protein are present in the sera of patients with ARF. First identified by Rebecca Lancefield in the 1920s, this α-helical coiled-coil protein (also known as the Lancefield antigen) projects through the capsule and onto the cell surface. It is structurally similar to tropomyosin and myosin found in heart tissues and other coiled-coil proteins, such as keratin, DNA, laminin, and vimentin. Once formed against the M protein, the antibodies cross-react with myosin, tropomyosin, vimentin, and laminin.2
The precise interplay between humoral and cellular immune responses in ARF and RHD is still being worked out, but it appears that streptococcal and heart cross-reactive antibodies bind the valvular endothelium. This action subsequently leads to inflammation of the heart tissue and upregulation of adhesion molecules, such as vascular cell adhesion molecule-1. In turn, this molecule attracts T cells that infiltrate valvular tissue and induce scarring and neovascularization, possibly allowing for further T-cell infiltration and cross-reactivity against newly exposed epitopes.2
In the main neurological manifestation of ARF, Sydenham chorea (also called Saint Vitus dance), it appears that antibrain antibodies react predominantly against the neurons of the basal ganglia. Once bound to their target, these antibodies induce dopamine release in the caudate nucleus, leading to the movement disorders characteristic of Sydenham chorea.3
GENETICS
As in many complex diseases, a person's susceptibility to ARF appears to be affected by a combination of environmental and genetic factors. There is a modest association between the gene products of the major histocompatibility complex and ARF. Studies have implicated HLA-DR7 as a susceptibility factor in many populations, HLA-DR2 in African Americans, and HLA-DR4 in white Americans.4
There is a stronger association between the proportion of D8/17 B cells and susceptibility to ARF. In a study conducted by Patarroyo and associates,5 the D8/17 antigen was expressed on the B cells of 71% to 74% of participants who had ARF, compared with only 17% of controls. Further studies showed that D8/17 expression is inherited in an autosomal recessive fashion, with incomplete penetrance.
Another study looked at the tissue distribution of the D8/17 antigen and noted its strong expression in cardiac, skeletal, and smooth muscle tissues. Subsequently, researchers identified a monoclonal antibody directed against D8/17 that also bound vimentin, myosin, and recombinant streptococcal M protein. Thus, the D8/17 antigen also may play a role in disease pathogenesis.6 As development of a vaccine against GAS progresses, a use for this antigen in altering the development of the disease and its sequelae is becoming increasingly apparent.
EPIDEMIOLOGY
Despite its decline in the United States and other developed nations, ARF remains a true threat that must be respected. In a national study conducted in 2000, ARF was seen in 14.8 cases per 100,000 hospitalized American pediatric patients. The highest rates of disease were observed in Utah, Hawaii, Pennsylvania, and New York.4
In addition, ARF continues to ravage developing nations under conditions of poverty, overcrowding, and limited access to antibiotics and medical care. Of the estimated 20 million cases of RHD that occur each year, 95% are in the developing world.7
In 2008, Tibazarwa and colleagues7 conducted a systemic review of prospective population-based studies on the incidence of the first attack of ARF. India was found to have the highest mean incidence, with an overall attack rate of 51 cases per 100,000 persons per year. In studies in Eastern Europe, the Middle East, Asia, and Australia, crude incidence rates and age-adjusted rates were greater than 10 cases per 100,000 persons per year. Rates in the United Sates, Chile, Martinique, Guadeloupe, and Sweden were 10 cases per 100,000 persons per year or lower.
In studies that were not restricted to the first incidence of ARF, documented disease rates were much higher. Notably, aboriginal children of central and northern Australia were shown to have disease rates as high as 245 to 351 cases per 100,000 persons per year. In addition, community-based surveillance estimates placed disease rates in these areas as high as 500 cases per 100,000 children per year.8
CLINICAL FEATURES
ARF typically lags behind streptococcal pharyngitis by 2 to 3 weeks. As a result, isolating GAS infection from the oropharynx at disease onset is rare. The peak onset is at age 6 to 20 years. About 70% of older children and young adults can recall an episode of pharyngitis; only 20% of younger children recollect having a sore throat.1 The disease onset most often is characterized by an acute febrile illness, accompanied by a large-joint migratory arthritis, possibly with concomitant signs of carditis and valvular inflammation or with CNS involvement and chorea. Although acute episodes typically are self-limited, they may lead to chronic and progressive heart disease and cardiac decompensation.
Arthritis
This is the most common symptom of ARF and often the first one to appear, especially in teenagers and young adults. The arthritis typically is a migratory, nondeforming, large-joint polyarthritis, especially of the knees, ankles, elbows, and wrists.
If the arthritis goes unmanaged, several joints are affected in quick succession, with each joint inflamed maximally for a few days to a week. The inflammation then decreases over the course of the week before disappearing; it typically lasts no longer than 4 weeks. Notably, the pain associated with this migratory arthritis often is much more pronounced than the objective signs of disease.
Physicians often manage the arthritis and arthralgia of ARF symptomatically early in their course. However, doing so may change the clinical presentation and deprive clinicians of seeing the characteristic signs of ARF.
In a study that looked at joint involvement in patients with ARF after they received treatment, involvement of a single large joint was common (25% of cases)1; the knee and ankle joints were affected most often. Less frequently affected were the elbow, wrist, and hip joints and the small joints of the feet (12% to 15% of cases).
Some diagnosticians differentiate the arthritis and arthralgia of ARF from those of poststreptococcal reactive arthritis (PSRA). The latter diagnosis typically is made in patients who have a history of GAS infection but present with arthritis atypical of ARF and without other systemic features. Whether PSRA is an entity distinct from ARF is debatable. However, a possible difference in the cause of this disease and that of ARF is suggested by differences in the latent period between infection and the onset of symptoms, a patient's response to NSAIDs, evidence of carditis, and extra-articular manifestations.1 PSRA most often begins within 7 to 10 days of exposure, responds poorly to anti-inflammatory agents, and occurs in the absence of other organ involvement.
Carditis
Cardiac involvement in ARF may include valvulitis, myocarditis, and pericarditis. Isolated mitral valve disease develops in nearly 60% of patients with carditis; combined mitral and aortic valve involvement is the next most common (Figure 1).
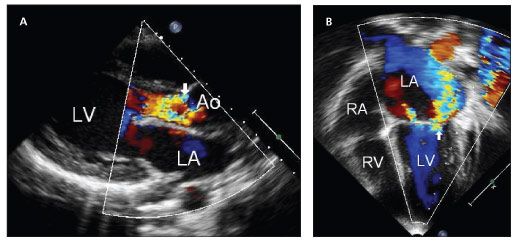
Figure 1 – Two-dimensional color and spectral Doppler echocardiographic studies of patients with rheumatic heart disease show moderate to severe aortic valve insufficiency with no stenosis (A, arrow) and bowing of the anterior mitral leaflet with severe insufficiency and no stenosis (B, arrow).
The pulmonic valve rarely is implicated. Valvular disease usually begins as valve dilatation with subsequent regurgitation. With chronic cardiac involvement, however, valvular regurgitation often leads to valvular stenosis.
Carditis typically presents clinically as a heart murmur, a pericardial rub, tachycardia or, in some cases, severe congestive heart failure (CHF). The most frequently heard murmur is a mitral regurgitant murmur. Less common are aortic regurgitant and systolic ejection murmurs. Estimates of the prevalence of carditis in ARF vary; some studies place it as low as 30% to 40%,4 and others suggest that more than 90% of patients with ARF have some degree of carditis.1 The estimates probably differ because of “subclinical carditis”-carditis that does not manifest clinically but may be detected with Doppler ultrasonography or with echocardiography.
Although many practicing physicians consider RHD to be clinically insignificant in the current era, it remains the primary cause of acquired valvular disease worldwide, often occurring 10 to 20 years after the initial episode of ARF. Mitral stenosis, the classic cardiac finding in RHD, presents clinically as a valvular murmur, cardiomegaly, or another sign of CHF.
Sydenham chorea
Much less common than carditis or arthritis, chorea occurs in 10% to 20% of patients with ARF.4 It typically is seen in older children and adolescents with ARF and occurs late in the disease course. This neurological disorder consists of involuntary, purposeless movements of the face and extremities. The patient is awake during these episodes and often experiences hemichorea or weakness, predominantly on 1 side of the body.
In addition, patients may be emotionally labile and demonstrate crying, grimacing, or restlessness. Speech often is slurred. These emotional and behavioral changes tend to manifest before the choreiform motor movements. The latent period for chorea typically is longer than that observed with the other manifestations of ARF and seldom is evident at the initial presentation. A strong association has been demonstrated between infection with GAS and obsessive-compulsive and tic disorders.9
Dermatological findings
The dermatological manifestations of ARF are uncommon and usually are self-limited. Erythema marginatum and subcutaneous nodules are the 2 most common findings.
Erythema marginatum is a nonpruritic, nonblanching, evanescent rash that occurs early in the disease process. It typically appears as small macules with a sharply defined outer edge that spreads centrifugally on the trunk and extremities, sparing the face.
The subcutaneous nodules of ARF usually are firm and nontender; they are found on the extensor surfaces of the wrists, elbows, knees, and ankles. Patients typically present with 3 to 4 nodules ranging in size from a few millimeters to 1 to 2 cm. They rarely persist for longer than 1 month. The nodules may be differentiated from those of rheumatoid arthritis (RA) in that they usually are smaller and short-lived. Although both often involve the elbows, the nodules of RA typically are found 3 to 4 cm distal to the olecranon; those of ARF lie directly over the bony projection.
Minor manifestations
ARF may be accompanied by several signs (eg, epistaxis [nosebleed], abdominal pain, rheumatic pneumonia, and fever) that are considered minor because they have a low rate of incidence. The fever is clinically defined as an oral or tympanic temperature of 38°C or higher; it usually decreases about 1 week into the disease course in the absence of treatment and rarely lasts longer than 3 to 4 weeks.
DIAGNOSIS
Patients who have ARF often present with a constellation of symptoms. Most physicians currently
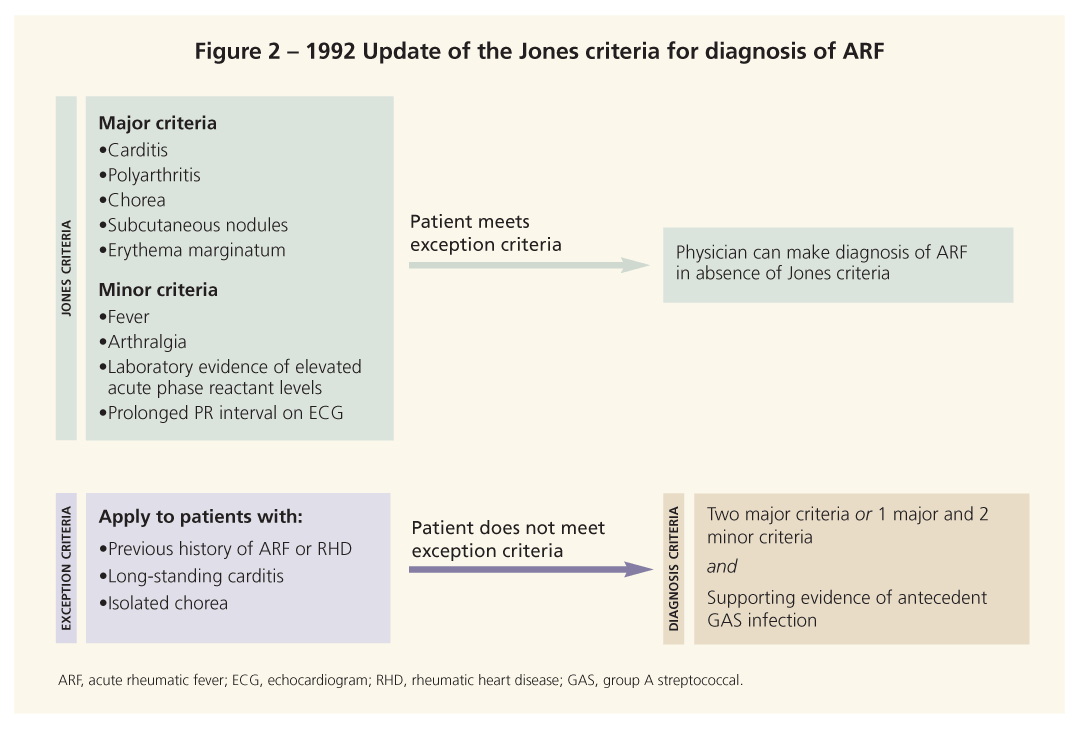
use the 1992 update of the Jones criteria to make the diagnosis (Figure 2).10,11 Although the criteria have been revised several times since they were created in 1944, their overall structure remains largely unchanged. Note, however, that each revision increased the specificity of the criteria but decreased the sensitivity. As a result, the World Health Organization (WHO) released its own, more sensitive criteria for the diagnosis of ARF.4
Jones criteria
These criteria divided the signs of ARF into major and minor ones. For a diagnosis of ARF, a patient must present clinically with 2 major manifestations or 1 major and 2 minor manifestations and supporting evidence of antecedent infection with GAS. However, providing evidence of GAS infection can be exceedingly difficult. Physicians may use a positive throat culture, a positive rapid streptococcal antigen test result, or an elevated or rising streptococcal antibody titer as evidence of infection. Antistreptolysin O titers are measured most often and are elevated in 80% of documented cases of ARF.1 Other clinically available antistreptococcal antibody tests include antihyaluronidase, anti-DNAse B, and anti-Streptozyme (a combination of several streptococcal antigens).10
The 1992 update of the Jones criteria cited situations in which patients may receive a diagnosis of ARF without meeting the aforementioned clinical guidelines for diagnosis. They include patients who have a past history of ARF or RHD, those who have long-standing carditis, and those who have isolated chorea.10 Recognizing these situations allows for rapid diagnosis and treatment of vulnerable patient populations.
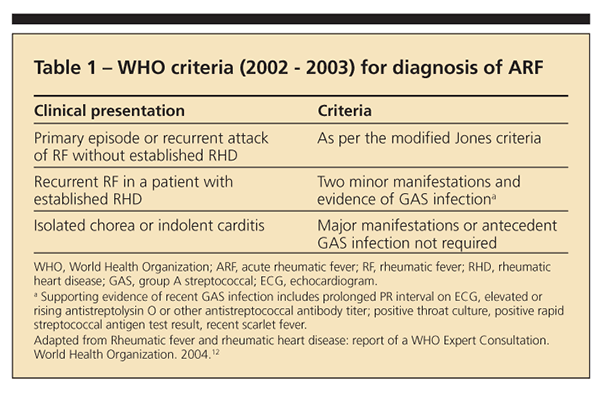
WHO criteria
Although similar to the Jones criteria, the WHO criteria are more sensitive and may be more useful in developing countries in avoiding underdiagnosis of ARF (Table 1).12 According to the WHO criteria, patients who present with isolated chorea or indolent carditis do not require evidence of antecedent GAS infection for the diagnosis to be made. Patients with established RHD require 2 minor manifestations plus evidence of antecedent GAS; the latter may be provided per the Jones criteria or through evidence of recent scarlet fever. In all other patient populations, the diagnosis should be made per the Jones criteria.4
Echocardiography
The role of Doppler echocardiography in the diagnosis of ARF is the subject of ongoing dialogue in the medical community. Although the importance of echocardiography has been acknowledged, this imaging modality has yet to be added to the Jones criteria.
The benefit of echocardiography lies in its sensitivity-it may be used to make a diagnosis of subclinical carditis or mild forms of carditis that do not produce audible murmurs. In an outbreak of ARF in Utah in 1985, 9 of 11 patients who had Sydenham chorea but no murmur on auscultation were shown to have mitral regurgitation. Similarly, 5 of 10 patients who had polyarthritis but no audible murmur were shown to have mitral regurgitation.13
In addition, a 1994 New Zealand study demonstrated the diagnostic potential of echocardiography in mixed valve lesions.14 Of 19 patients with valvular regurgitation, 12 were shown by echocardiography to have regurgitation in valves beyond those suspected clinically.
Whether the diagnosis and management of subclinical carditis affects prognosis is still unknown. In addition, extensive use of echocardiography runs the risk of overdiagnosis of ARF. Trivial regurgitation is common in both the mitral and tricuspid valves, and a skilled technician is needed to differentiate between physiological and pathological leaks.10 The role of echocardiography in the diagnosis of ARF will surely continue to evolve.
TREATMENT
As in any complex disease, the management of ARF should be tailored to the patient's unique set of symptoms. Many of the treatments for patients with ARF have not been tested in randomized controlled trials.
As a rule, if GAS is still demonstrable when the patient presents symptomatically, physicians should provide treatment with oral penicillin (or, if patient compliance issues are suspected, a single intramuscular [IM] injection of benzathine benzylpenicillin). Antimicrobial therapy does not alter the course, frequency, or severity of cardiac involvement.
Arthralgia, arthritis, and fever are best managed with salicylates or NSAIDs. Traditionally, 80 to 100 mg/kg/d of aspirin is given to children and 4 to 8 g/d to adults for the first 2 weeks of treatment. Salicylates should not be used to manage carditis.
In severe cases of rheumatic carditis, corticosteroids may be added as a means to reduce the inflammatory response. Prednisone, the preferred corticosteroid, is given at 1 to 2 mg/kg/d initially, with a maximum dosage of 80 mg/d. It then can be tapered by 20% to 25% over a 2- to 3-week period.
Chorea typically is benign and self-limited and does not require treatment. More protracted cases may be managed with haloperidol.
Patients in whom carditis and subsequent CHF develop need to be treated medically with inotropic agents (eg, digoxin), diuretics, and afterload reduction in addition to receiving treatment for the inflammatory response. If RHD develops, a more aggressive approach may be required.
In the absence of significant mitral calcification or regurgitation, mitral stenosis may be managed with percutaneous balloon mitral valvuloplasty.15 When that is not possible, the mitral valve may need to be replaced. In the presence of mitral stenosis with aortic valve disease or atrial fibrillation or both, surgical intervention should be approached with much greater caution. In combined mitral and aortic valve disease, outcomes were better with a combined mitral valve repair and aortic valve replacement than with a double valve replacement.15
PREVENTION
So great is the worldwide prevalence of GAS infection and its sequelae that efforts should be focused on prevention. GAS infection-the leading cause of bacterial pharyngitis-is responsible for an estimated 15% to 30% of acute cases, leading to 663,000 new cases and 163,000 deaths each year. In addition, an estimated 15.6 million persons worldwide are currently living with RHD and 460,000 new diagnoses are made each year.
Current prevention efforts are focused on primary and secondary prophylaxis (Table 2). For primary
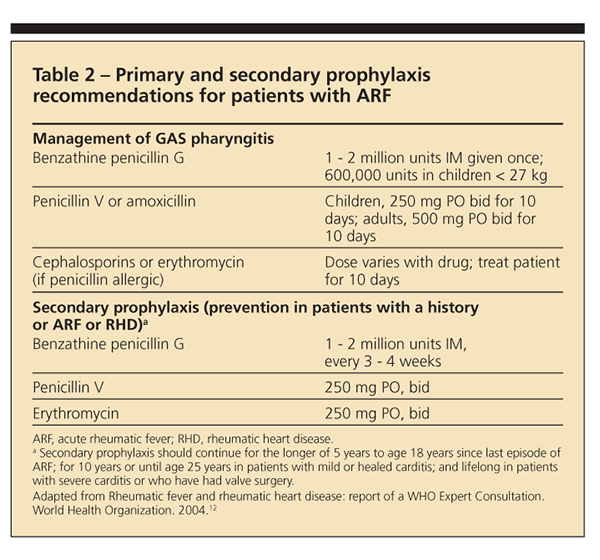
prophylaxis, an antibiotic given within 9 days of sore throat caused by GAS infection prevents most cases of ARF. Physicians are encouraged to treat their patients with IM benzathine benzylpenicillin or, less preferable, 10 days of penicillin V. If the patient is allergic to the penicillin class of drugs, erythromycin may be used. In regions where the burden of disease is high, however, primary prophylaxis is neither cost-effective nor practical.
For secondary prophylaxis of patients with a history of ARF or RHD, physicians are encouraged to give IM benzathine benzylpenicillin every 3 to 4 weeks. Although oral penicillin taken twice daily is another option for prophylaxis, physicians run the risk of patient noncompliance. The length of treatment is variable and based on the patient's risk of recurrence. Current recommendations are for persons to receive prophylactic therapy for at least 10 years after the last episode of ARF and until at least age 40 years.
Because the prevalence of ARF and RHD is high in developing countries, vaccination may be the most practical and cost-effective option for disease prophylaxis. Several streptococcal antigens have been studied as potential vaccine targets, although the most current research seems focused on vaccines against the streptococcal M protein.
The first promising vaccine is a subunit vaccine targeted against the hypervariable region of the M protein. By using recombinant proteins, scientists hope to remove potentially cross-reactive epitopes. This vaccine structure also allows them to combine various aminoterminal epitopes from the M protein into a single, multivalent target.
References:
References1. Gibofsky A, Zabriskie JB. Poststreptococcal arthritis and rheumatic fever. In: Firestein GS, Budd RC, Harris ED, et al, eds. Kelley's Textbook of Rheumatology. 8th ed. Philadelphia: WB Saunders; 2009:1771-1783.
2. Faé KC, da Silva DD, Oshiro SE, et al. Mimicry in recognition of cardiac myosin peptides by heart-intralesional T cell clones from rheumatic heart disease. J Immunol. 2006;176:5662-5670.
3. Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9:914-920.
4. Miyake CY, Gauvreau K, Tani LY, et al. Characteristics of children discharged from hospitals in the United States in 2000 with the diagnosis of acute rheumatic fever. Pediatrics. 2007;120:503-508.
5. Patarroyo ME, Winchester RJ, Vejerano A, et al. Association of a B-cell alloantigen with susceptibility to rheumatic fever. Nature. 1979;278:173-174.
6. Bryant PA, Robins-Browne R, Carapetis JR, Curtis N. Some of the people, some of the time: susceptibility to acute rheumatic fever. Circulation. 2009;119:742-753.
7. Tibazarwa KB, Volmink JA, Mayosi BM. Incidence of acute rheumatic fever in the world: a systematic review of population-based studies. Heart. 2008;94:1534-1540.
8. Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366:155-168.
9. Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases [published correction appears in Am J Psychiatry. 1998;155:578]. Am J Psychiatry. 1998;155:264-271.
10. Saxena A. Diagnosis of rheumatic fever: current status of Jones Criteria and role of echocardiography. Indian J Pediatr. 2000;67(3 suppl):S11-S14.
11. Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. JAMA. 1992;268:2069-2073.
12. WHO. Rheumatic fever and rheumatic heart disease: report of a WHO Expert Consultation. Geneva, 29 October-1 November 2001. Geneva: World Health Organization; 2004.
13. Veasy LG, Wiedmeier SE, Orsmond GS, et al. Resurgence of acute rheumatic fever in the intermountain area of the United States. N Engl J Med. 1987;316:421-427.
14. Abernethy M, Bass N, Sharpe N, et al. Doppler echocardiography and the early diagnosis of carditis in acute rheumatic fever. Aust N Z J Med. 1994;24:530-535.
15. Zakkar M, Amirak E, Chan KM, Punjabi PP. Rheumatic mitral valve disease: current surgical status. Prog Cardiovasc Dis. 2009;51:478-481.




