Article
Latent Tuberculosis Infection in RA: Current Diagnostic Techniques and Treatment
The risk of latent tuberculosis infection (LTBI) is greater in patients with rheumatoid arthritis (RA). Regular screening for LTBI is recommended.
ABSTRACT: The risk of latent tuberculosis infection (LTBI) is greater in patients with rheumatoid arthritis (RA). Regular screening for LTBI is recommended. The Interferon-Gamma Release Assays (IGRAs) overcome some of the disadvantages of the tuberculin skin test. Managing LTBI reduces the risk of progression to active tuberculosis (TB), especially in high-risk groups such as those with RA. Thus, early detection and treatment are the mainstays for decreasing the morbidity and mortality resulting from TB in patients with RA. Isoniazid treatment for patients with LTBI was first recommended for general use in 1965. Rifampin-based regimens might be safer, more effective, and shorter. Patients should be educated about the adverse effects associated with treatment. A 2-step testing approach has been suggested. (J Musculoskel Med. 2011;28:300-309)
The risk of latent tuberculosis infection (LTBI) is far greater in patients with rheumatoid arthritis (RA) than in the general population, and regular screening of these patients for LTBI is recommended. Traditionally, the diagnosis has been based on tuberculin skin test (TST) results as well as on information gathered from the medical history, chest radiography, and physical examination. In recent years, however, newer diagnostic techniques have been developed that may have advantages over the TST.
This 2-part article offers 20 “clinical pearls” to answer key questions about LTBI in patients with RA. In the first part (“Latent tuberculosis infection in RA: The disease and the diagnosis,”The Journal of Musculoskeletal Medicine, July 2011, page 249), we described the traditional diagnostic tests and interpretation of their results. This second part discusses the newer diagnostic techniques, provides a review of the current literature, and describes the current guidelines for treatment and monitoring.
10. What are the newer techniques for the diagnosis of LTBI?
The Interferon-Gamma Release Assays (IGRAs), a newer technique, are based on the principle that sensitized T cells release interferon γ (IFN-γ) when exposed to Mycobacterium tuberculosis antigens.1 The IGRAs include the QuantiFERON-TB Gold (QFT-G) test and the T-SPOT TB (ELISPOT) test. The TST and IGRAs evaluate cell-mediated immunity.
Several studies have addressed the usefulness of IGRAs in patients with RA.2-9 IFN-γ assays that use M tuberculosis–specific region of difference 1 antigens, such as early secretory antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10), have advantages over the TST.1,10-12
The QFT-G test was approved in 2001. The T-SPOT TB test, approved more recently, was incorporated into the CDC guidelines for use of IGRAs updated in June 2010. The guidelines recommend the use of IGRAs preferentially in patients who are BCG-vaccinated and those who are unlikely to return for a TST reading.13
IGRAs overcome some of the disadvantages of the TST, as follows:
• In patients with RA, the results do not change because of their immune status.
• A patient requires only 1 visit to the health care facility, and the results are available in a day.
• There is computerized reading of the tests, decreasing the chances of human errors.
• They have higher specificity-the result is positive only with M tuberculosis,1,10 unlike with the TST.
Limitations include cost and not being widely available. Also, there is a relatively high number of indeterminate results (up to 12%).
11. What is the difference between the QFT-G test and the T-SPOT TB test?
The QFT-G test measures IFN-γ secretion by sensitized T cells after in vitro stimulation with M tuberculosis–specific antigens (ESAT-6, CFP-10, TB7-7) and uses whole blood for measurement. The T-SPOT TB test measures the number of sensitized T cells that secrete IFN-γ and uses peripheral blood mononuclear cells (PBMCs).
12. What are the T-SPOT TB test methods?
Isolated lymphocytes are sensitized to M tuberculosis antigens, and the activated T cells produce IFN-γ. The T-SPOT TB test uses a simplified ELISPOT method to enumerate sensitized T cells by capturing IFN-γ in the vicinity of those cells from which it was secreted.
Washed and counted PBMCs are seeded into 4 microtiter wells where they are exposed to phytohemagglutinin, a mitotic stimulator that indicates cell functionality (positive control); a nil control; and 2 separate panels of M tuberculosis–specific antigens. Secreted cytokine is captured by IFN-γ–specific antibodies in the base of the well and detected with a second antibody that is linked to a color detection agent. Evaluating the number of spots obtained provides a measure of the abundance of M tuberculosis–sensitive effector T cells in the peripheral blood (0 to 4 spots, negative; 5 to 7 spots, equivocal; more than 8 spots, positive).14
13. What are the differences among the QuantiFERON tests?
The FDA approved the QuantiFERON-TB assay for the diagnosis of LTBI in 2001.11 This test, which measured IFN-γ after whole blood stimulation by the purified protein derivative (PPD) from M tuberculosis, is now off the market. It was replaced by the QFT-G test, where PPD was replaced by the ESAT-6 and CFP-10 antigens to avoid cross-reactions with nontuberculous mycobacteria or Calmette-Gurin bacilli. This test was approved by the FDA in 2004.15 The third antigen, TB7.7, was added to the QFT-G test soon after. The QFT-G test was simplified later with direct addition of the antigens in-tube (IT). Called QFT-G IT, the test received FDA approval in 2007.
14. Describe the QFT-G test.
The test requires 5 mL of heparinized whole blood incubated for 16 to 24 hours at 37°C (99°F) in a humidified atmosphere. The test has a negative control (nil well, which has whole blood without antigens or mitogens); a positive control (mitogen well, which has whole blood stimulated with mitogen phytohemagglutinin); and 2 sample wells, which have whole blood with M tuberculosis–specific antigens.
IFN-γ levels in the nil well are subtracted from the results of the mitogen and the sample wells because they are considered background for the same. The results are considered positive if the IFN-γ concentration in the sample wells is 0.35 IU/mL or greater (after subtraction of the nil well value) and negative if the IFN-γ concentration in the sample wells is less than 0.35 IU/mL (after subtraction of the nil well value) and if the positive control is 0.5 IU/mL or greater. The results are considered indeterminate if both antigen-stimulated sample wells are negative and if the value of the positive control well is less than 0.5 IU/mL (after subtraction of the nil well value).
A positive result suggests that M tuberculosis infection is likely, and a negative result suggests that infection is unlikely. An indeterminate result suggests that the QFT-G test results cannot be interpreted because of low mitogen response or high background response.
15. What are the disadvantages of the QFT-G test?
Blood samples must be processed within 12 hours after collection while white blood cells are still viable. There are limited data on the applicability of the QFT-G test in screening children (younger than 17 years), persons recently exposed to M tuberculosis infection, and immunocompromised persons (those with HIV/AIDS, some hematological disorders, malignancies, diabetes mellitus, silicosis, or chronic kidney disease and those currently receiving treatment with immunosuppressive drugs). Errors in collecting or transporting blood specimens or in running and interpreting the assay can decrease the accuracy of the QFT-G test. Data on the use of QFT-G to determine who is at risk for tuberculosis (TB) are limited.
The QFT-G test cannot differentiate between active and latent infection, which decreases its specificity, and impaired cellular response consequent to active TB diminishes its sensitivity. Discrepancies between the TST and IGRAs include the following: positive TST results but negative IGRA results are associated with previous BCG vaccination, and negative TST results but positive IGRA results are associated with corticosteroid therapy.
16. What are the differences between the TST and IGRAs?
There are several differences. They are outlined in Table 1.
17. What does the current literature say about diagnosis of LTBI in patients with RA?
TABLE 1
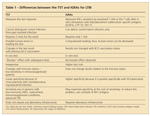
Differences between the TST and IGRAs for LTBI
The T-SPOT TB and QFT-G are more sensitive in detecting LTBI in patients with RA, according to several studies, including those by Bocchino and associates8 and Martin and colleagues.16 It has been suggested that the QFT-G can detect LTBI in patients with RA but the TST may give false-negative results (Takahashi and coworkers,3 Matulis and associates,17 Ponce de Leon and colleagues,7 Efthimiou and Sood,5 and Gogus and coworkers18). In addition, it has been suggested that the T-SPOT TB test performs better than the TST in patients with RA by identifying patients with LTBI in spite of a false-negative TST result (Girlanda and colleagues,19 Vassilopoulos and associates,20 and Sellam and coworkers9). Contrary to those findings, Shovman and colleagues21 argued that the high rates of indeterminate results may limit the clinical utility of the QFT-G in the diagnosis of LTBI in patients with RA.
Ponce de Leon and coworkers7 reported that agreement between QFT-IT and TST results is poor in patients with immune-mediated inflammatory diseases, such as RA, and better in healthy controls. Therefore, the TST may not be the best choice for LTBI detection in patients with RA even though it is appropriate for population screening. The higher rate of indeterminate results for the QFT-G in immunocompromised patients than in the general population suggests that as in the TST, a lower cutoff for positivity of the QFT-G may be needed in immunocompromised patients to improve sensitivity and positive predictive value.22
Vassilopoulos and coworkers20 suggested that corticosteroid use (mean dose, 6.8 mg/d of prednisolone) is associated with TST-negative/ELISPOT-positive results, making the latter test especially useful in RA, where patients are still treated long-term with corticosteroids. Matulis and associates17 collected data from 142 consecutive patients with immune-mediated inflammatory diseases, including 40 patients with RA; their results showed that the QFT-IT performs better than the TST for detection of LTBI in patients who are receiving immunosuppressive therapy.
Of note, anti–tumor necrosis factor α (anti–TNF-α) treatment was associated with lower odds of IGRA positivity than were corticosteroids or traditional disease-modifying antirheumatic drugs. This finding may reflect intensified screening and treatment of patients for LTBI before the initiation of TNF-α inhibitor therapy rather than a direct inhibition by the TNF-α inhibitors of the activation of CD4+ lymphocytes by Mycobacterium antigens, leading to false-negative testing. The authors went on to suggest that the combination of the TST with an IGRA may be advantageous in these cases.
18. What are the current treatment guidelines for patients who have LTBI with RA?
The current literature suggests that managing LTBI reduces the risk of progression to active TB, especially in high-risk groups, such as those with RA and patients who are receiving immunosuppressive medications.23,24 Thus, early detection and treatment are the mainstays for decreasing the morbidity and mortality resulting from TB in patients with RA.25
TST- or IGRA-positive contacts who can provide written documentation of previous adequate treatment for LTBI generally do not need to be re-treated. However, re-treatment may be indicated for persons at high risk for becoming reinfected and having their disease progress to active TB or for having a positive IGRA result.
Isoniazid treatment for patients with LTBI was first recommended for general use by the American Thoracic Society in 1965.26 In 1970, among several thousand persons who began isoniazid treatment as a result of an outbreak of TB on Capitol Hill in the District of Columbia, clinical signs of liver disease developed in 19 persons and 2 persons died of hepatic failure that was attributed to the use of isoniazid.27
Because there were concerns about actual and perceived isoniazid toxicity and patient nonadherence with the relatively long treatment period that was required, alternatives to isoniazid were suggested (Table 2).28 The introduction of rifampin, which appeared to be a better sterilizing agent than isoniazid, suggested that rifampin-based regimens might be safer, more effective, and shorter. The HIV epidemic and the need to evaluate the efficacy of treatment for LTBI in persons coinfected with HIV and M tuberculosis led to a series of studies of short-course treatment of LTBI in HIV-infected patients.29
TABLE 2

Isoniazid vs rifampin for managing LTBI
Health departments or health care providers may conclude, on the basis of local conditions, that a 6-month rather than a 9-month course of isoniazid is preferred (Table 3). For pregnant, HIV-negative women, isoniazid given daily or twice weekly for 9 or 6 months is recommended. For women at risk for progression of LTBI to disease, especially those who are infected with HIV or who probably have been infected recently, the start of therapy should not be delayed on the basis of pregnancy alone, even during the first trimester. For women whose risk of active TB is lower, some experts recommend waiting until after delivery to start treatment.
19. Monitoring of treatment for patients with LTBI.
Once patients have been identified and have tested positive for LTBI, they should receive an initial clinical evaluation. After the start of treatment, they should receive monthly follow-up evaluations if they are receiving isoniazid or rifampin monotherapy. Evaluations should include questioning about adverse effects and a brief physical assessment to check for signs of hepatitis.
TABLE 3
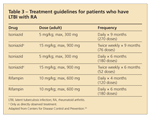
Treatment guidelines for patients who have
Patients should be educated about the adverse effects associated with treatment and advised to stop treatment and promptly seek medical evaluation if they occur.30 In a study conducted by Mor and colleagues,31 the use of isoniazid for LTBI was well tolerated in patients with RA who were treated with methotrexate and in whom the added hepatotoxicity risk with isoniazid appeared to be low. However, closely monitoring serum aminotransferase levels in patients taking this combination is prudent.
20. Testing recommendations and regional variations in LTBI screening.
When the advantages and disadvantages of the TST and IGRAs are weighed, IGRAs appear to be superior tools for the diagnosis of LTBI in patients with RA. However, data for patients with RA are still insufficient to recommend one particular IGRA over another. To further complicate things, the use of a 2-step approach (screening with the TST and confirmation with IGRAs) has been suggested to be cost-effective and more sensitive in some at-risk populations, such as close contacts of infected patients; this still needs to be proved convincingly in patients with RA.
A lack of definitive evidence explains differences in screening guidelines in various countries. A single IGRA can replace the TST, according to the CDC,15 but the UK-based National Institute for Health and Clinical Excellence32 and revised Canadian guidelines33 both recommend a 2-step strategy to perform IGRA only for TST-positive persons. Treatment with antituberculous agents is recommended for all patients who have latent infection.25
References:
References
1. Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099-1104.
2. Pratt A, Nicholl K, Kay L. Use of the QuantiFERON TB Gold test as part of a screening programme in patients with RA under consideration for treatment with anti-TNF-alpha agents: the Newcastle (UK) experience. Rheumatology (Oxford). 2007;46:1035-1036.
3. Takahashi H, Shigehara K, Yamamoto M, et al. Interferon gamma assay for detecting latent tuberculosis infection in rheumatoid arthritis patients during infliximab administration. Rheumatol Int. 2007;27:1143-1148.
4. Kaushik VV, Ambalavanan S, Binymin K. Comment on: Use of the QuantiFERON TB Gold test as part of a screening programme in patients with RA under consideration for treatment with anti-TNF-alpha agents: the Newcastle (UK) experience. Rheumatology (Oxford). 2007;46:1863-1864.
5. Efthimiou P, Sood S. QuantiFERON TB Gold Test: the new standard for screening of latent tuberculosis in patients with rheumatoid arthritis? Ann Rheum Dis. 2007;66:276.
6. Greenberg JD, Reddy SM, Schloss SG, et al. Comparison of an in vitro tuberculosis interferon-gamma assay with delayed-type hypersensitivity testing for detection of latent Mycobacterium tuberculosis: a pilot study in rheumatoid arthritis [published correction appears in J Rheumatol. 2008;35:943]. J Rheumatol. 2008;35:770-775.
7. Ponce de Leon D, Acevedo-Vasquez E, Alvizuri S, et al. Comparison of an interferon-gamma assay with tuberculin skin testing for detection of tuberculosis (TB) infection in patients with rheumatoid arthritis in a TB-endemic population. J Rheumatol. 2008;35:776-781.
8. Bocchino M, Matarese A, Bellofiore B, et al. Performance of two commercial blood IFN-gamma release assays for the detection of Mycobacterium tuberculosis infection in patient candidates for anti-TNF-alpha treatment. Eur J Clin Microbiol Infect Dis. 2008;27:907-913.
9. Sellam J, Hamdi H, Roy C, et al; RATIO (Research Axed on Tolerance of Biotherapies) Study Group. Comparison of in vitro-specific blood tests with tuberculin skin test for diagnosis of latent tuberculosis before anti-TNF therapy. Ann Rheum Dis. 2007;66:1610-1615.
10. Lalvani A. Spotting latent infection: the path to better tuberculosis control. Thorax. 2003;58:916-918.
11. Mazurek GH, Villarino ME. Guidelines for using the QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52(RR-2):15-18.
12. Lalvani A. Counting antigen-specific T cells: a new approach for monitoring response to tuberculosis treatment? Clin Infect Dis. 2004;38:757-759.
13. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection-United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
14. Principles of the T-SPOT Assay. Oxford Immunotec. 2010. http://www.oxfordimmunotec.com/How_It_Works_International. Accessed June 6, 2011.
15. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States [published correction appears in MMWR Morb Mortal Wkly Rep. 2005;54:1288]. MMWR Recomm Rep. 2005;54(RR-15):49-55.
16. Martin J, Walsh C, Gibbs A, et al. Comparison of interferon {gamma} release assays and conventional screening tests before tumour necrosis factor {alpha} blockade in patients with inflammatory arthritis. Ann Rheum Dis. 2010;69:181-185.
17. Matulis G, Jüni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008;67:84-90.
18. Gogus F, Günendi Z, Karakus R, et al. Comparison of tuberculin skin test and QuantiFERON-TB gold in tube test in patients with chronic inflammatory diseases living in a tuberculosis endemic population. Clin Exp Med. 2010;10:173-177.
19. Girlanda S, Mantegani P, Baldissera E, et al. ELISPOT-IFN-gamma assay instead of tuberculin skin test for detecting latent Mycobacterium tuberculosis infection in rheumatic patients candidate to anti-TNF-alpha treatment. Clin Rheumatol. 2010;29:1135-1141.
20. Vassilopoulos D, Stamoulis N, Hadziyannis E, Archimandritis AJ. Usefulness of enzyme-linked immunospot assay (Elispot) compared to tuberculin skin testing for latent tuberculosis screening in rheumatic patients scheduled for anti-tumor necrosis factor treatment. J Rheumatol. 2008;35:1271-1276.
21. Shovman O, Anouk M, Vinnitsky N, et al. QuantiFERON-TB Gold in the identification of latent tuberculosis infection in rheumatoid arthritis: a pilot study. Int J Tuberc Lung Dis. 2009;13:1427-1432.
22. Manuel O, Kumar D. QuantiFERON-TB Gold assay for the diagnosis of latent tuberculosis infection. Expert Rev Mol Diagn. 2008;8:247-256.
23. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis: a general review. Bibl Tuberc. 1970;26:28-106.
24. Whalen CC, Johnson JL, Okwera A, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda-Case Western Reserve University Research Collaboration. N Engl J Med. 1997;337:801-808.
25. Carmona L, Gómez-Reino JJ, RodrÃguez-Valverde V, et al; BIOBADASER Group. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52:1766-1772.
26. Runyon EH. Preventive treatment in tuberculosis: a statement by the Committee on Therapy, American Thoracic Society. Am Rev Respir Dis. 1965;91:297-298.
27. Garibaldi RA, Drusin RE, Ferebee SH, Gregg MB. Isoniazid-associated hepatitis: report of an outbreak. Am Rev Respir Dis. 1972;106:357-365.
28. Snider DE Jr, Farer LS. Preventive therapy for tuberculous infection: an intervention in need of improvement. Am Rev Respir Dis. 1984;130:355-356.
29. O'Brien RJ, Perriëns JH. Preventive therapy for tuberculosis in HIV infection: the promise and the reality. AIDS. 1995;9:665-673.
30. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4, pt 2):S221-S247.
31. Mor A, Bingham CO 3rd, Kishimoto M, et al. Methotrexate combined with isoniazid treatment for latent tuberculosis is well tolerated in patients with rheumatoid arthritis: experience from an urban arthritis clinic. Ann Rheum Dis. 2008;67:462-465.
32. Wiselka M; Royal College of Physicians; British Infection Society on the NICE TB Guidelines Committee. 2006: a year for cautious optimism in tuberculosis. J Infect. 2006;53:1-4.
33. Canadian Tuberculosis Committee. Interferon gamma release assays for latent tuberculosis infection: an Advisory Committee Statement (ACS). Can Commun Dis Rep. 2007;33(ACS-10):1-18.
34. Latent Tuberculosis Infection: A Guide for Primary Health Care Providers. Treatment of Latent TB Infection. Centers for Disease Control and Prevention. 2010. http://www.cdc.gov/tb/publications/LTBI/treatment.htm#2. Accessed June 6, 2011.




