Article
Limited Joint Mobility in Diabetes Mellitus: The Clinical Implications
Limited joint mobility (LJM) is a common complication of diabetes mellitus (DM). LJM often is characterized by hand stiffness, but other joints may be involved. The prayer and tabletop signs may be used to detect limitation of joint mobility in the hands.
ABSTRACT: Limited joint mobility (LJM) is a common complication of diabetes mellitus (DM). LJM often is characterized by hand stiffness, but other joints may be involved. The prayer and tabletop signs may be used to detect limitation of joint mobility in the hands. Range of motion should be checked in the large joints as well as in the hand and finger joints. LJM should be distinguished from other musculoskeletal conditions that also are seen frequently in the hands of patients with DM. LJM may be associated with the duration of DM. Treatment is controversial. Strict control of blood glucose usually is advocated. Physical therapy may improve function. No medications have been approved for clinical use. (J Musculoskel Med. 2011;28:118-124)
Limited joint mobility (LJM), or diabetic cheiroarthropathy, is a condition characterized by hand stiffness resulting from flexion contractures of the fingers and by thickened, tight, waxy skin.1 “LJM” is the newer, preferred term used in describing the condition because joints other than those in the hands (eg, in the wrists and elbows, feet, and spine) also may be involved.2
Lundbaek3 first reported LJM in 5 patients with diabetes mellitus (DM) in 1957, but the syndrome did not receive more attention until 1974, when Rosenbloom and Frias4 described it again in children with DM. The existence of this clinical entity was confirmed by larger studies of children with insulin-dependent (type 1) DM5-7 and, subsequently, was demonstrated in adult and geriatric patients with non–insulin-dependent (type 2) DM.8-12
LJM is a common complication of DM, occurring in 8% to 58% of patients; most studies suggest that the prevalence is about 30% to 40%.1,7,13,14 Although early investigators did not find sex differences, one study reported that adolescents who have DM with LJM are predominantly male.15 No racial differences have been found.
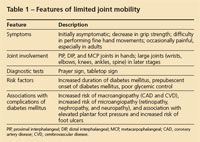
The onset of LJM is insidious and may predate the recognition of overt DM (Table 1). Although there is moderate limitation of finger joint mobility, LJM usually is neither painful nor disabling. In making the diagnosis, it is important to differentiate LJM from other DM-related hand conditions. LJM has been linked to poor glycemic control and other complications of DM in retrospective studies, but whether it predates the appearance of renal or ophthalmic disease and whether careful blood glucose control with insulin therapy can reduce the rate of its development has not been conclusively determined. However, quick and easy office assessment for LJM should be a part of the routine assessment of patients with DM, and its presence should alert the physician to the likely presence of microvascular or macrovascular disease or both.
In this article, we describe the diagnosis and differential diagnosis of LJM, the physical examination and testing, and the association of LJM with DM and various musculoskeletal conditions. We also discuss the controversies involved in treatment of patients with this condition.
Clinical course
In patients with LJM, asymptomatic contractures first develop in the distal interphalangeal and proximal interphalangeal (PIP) joints and, eventually, spread to the metacarpophalangeal (MCP) joints. In the early stages, most patients are completely asymptomatic. With time, however, both handgrip strength and ability to perform fine movements decrease, and patients complain of stiffness, weakness, clumsiness, and decreased dexterity. Gradually, the joint contractures may extend beyond the hands to the larger joints, including the elbows, shoulders, knees, and axial skeleton.
Typically, LJM is painless, especially in juvenile patients with DM. However, adult patients with DM may have coexisting neuropathy and may report pain.
Diagnosis
A diagnosis of LJM is made with careful physical examination and exclusion of other conditions in the differential diagnosis. The index of suspicion should be high in all patients with DM, especially those who have had the disease for a long duration. Results of laboratory and radiographic evaluation usually are nonspecific and unremarkable. The erythrocyte sedimentation rate is normal; antinuclear antibodies and rheumatoid factor typically are absent.16 The results of nail fold capillaroscopy usually are normal.17,18
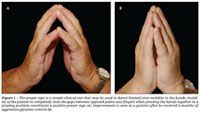
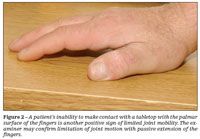
Two simple clinical tests may be used to detect limitation of joint mobility in the hands. In the prayer sign, the patient is asked to put his or her hands together in a praying position with the fingers fanned and to press together the palmar surfaces of the interphalangeal joints and the palms (Figure 1).7 The tabletop test is conducted by asking the patient to place his hands palms-down on a tabletop with the fingers spread (Figure 2).5 The examiner views the fingers at the table level to determine their degree of contact with the table. In both tests, the entire palmar surface of the fingers making contact constitutes a normal result. If contact with the palmar surface is incomplete, the examiner may confirm limitation of joint motion with passive extension of the fingers.
Sauseng and associates19 compared the use of the prayer sign with the use of a goniometer in measuring the exact range of motion of the hand joints. They found that both approaches correlate well in their ability to detect LJM in patients with DM. With overenthusiastic use of the prayer sign, however, abnormalities may be detected in many normal patients. In one study, 26% of healthy controls were found to have a positive prayer sign.13
A patient’s range of motion should be checked in the large joints as well as in the hand and finger joints. Joint limitation may be classified as9:
•Slight, if there is impaired extension of either the PIP or MCP joints.
•Moderate, if there is reduced extension of both the PIP and MCP joints.
•Severe, if there is impaired extension of the PIP and MCP joints and limitation of a large joint.
Differential diagnosis
LJM should be distinguished from other musculoskeletal conditions that also are seen frequently in the hands of patients with DM and may confuse the clinical picture. They include the following:
•Dupuytren contracture. This is a contracture of the digits associated with a nodular and palpably thickened palmar fascia and dimpling or retraction of the overlying palmar skin. Usually, it is asymmetrical, but it tends to involve the fourth and fifth digits first.
•Tenosynovitis of the finger flexor tendons. This is thickening or nodularity of a tendon sheath without dimpling or traction of the overlying palmar skin. Tenosynovitis may be complicated by trigger finger, an observable flexion contracture of an individual digit that reduces abruptly with forced extension, resulting in acute pain in the region of the corresponding palmar flexor tendon.
•Reflex sympathetic dystrophy. This condition is characterized by pain, diffuse swelling, pitting edema, and regional temperature changes. A bone scan may demonstrate characteristic flow abnormalities.
All 3 conditions may coexist in a single patient, because all occur more frequently in patients with DM than in the normal population. Other conditions that have a clinical appearance similar to that of LJM include the following:
•Palmar fasciitis/fibromatosis. This rare disorder is characterized by tenderness and induration of the palmar aponeurosis with nodules and rapidly progressive flexion contractures of the fingers; usually, it affects all the digits of both hands. The changes are similar to those of Dupuytren contracture but are more widespread. The progressive changes lead to an appearance and texture of “woody hands.” The disorder may be associated with an aggressive inflammatory polyarthritis in the hands-palmar fasciitis and polyarthritis syndrome-a paraneoplastic syndrome associated with several malignant neoplasms. Initially, it was described in ovarian cancer; later, it also was found with other malignancies, including pancreatic and breast cancer. Usually, there is no sclerosis of the dermis.
•Scleroderma. This multisystem connective-tissue disease is characterized by thickening of the skin of the fingers and hands that may spread proximally. Often, it is associated with the presence of autoantibodies, Raynaud phenomenon, and abnormal nail fold capillaries. Other factors that may help distinguish scleroderma from LJM include the presence of telangiectasia and calcinosis.
Pathogenesis
The development of LJM is complex and multifactorial. Factors that may lead to impaired mobility include alterations of the structures in the hand (eg, the intrinsic muscles, joint capsule, subcutaneous tissue, and skin). These changes may result from the interaction of vascular ischemia and alterations in the structure or composition of collagen.
One consequence of prolonged hyperglycemia is nonenzymatic glycosylation of collagen analogous to glycosylated hemoglobin (HbA1c).20 This glycosylation results in abnormally cross-linked collagens, which are unusually resistant to mechanical and enzymatic degradation,21 and collagen accumulation in the connective tissue of patients with DM.14,22 This glycosylated collagen also may entrap potentially harmful nonglycosylated proteins (eg, albumin, immunoglobulins, and coagulation proteins) and contribute to increased extracellular matrix accumulation.20 Glycosylated collagen has been shown to be antigenic in mice and may induce an antibody response.23
Nonenzymatic glycosylation of collagen also disturbs the cellular and structural components of the microvasculature, resulting in thickening of the capillary basement membrane. This is the fundamental morphological change in diabetic microangiopathy,24,25 which may then directly contribute to fibrosis by inducing low-grade ischemia and chronic tissue injury.26 Injury to connective tissue also may be mediated by excessive flux through the aldose reductase pathway, resulting in depletion of essential osmolytes, cytotoxic edema, and membrane injury.27
Early glycosylation of skin collagen may be decreased with improved glycemic control. However, the long-term cumulative damage that may result from the binding of advanced glycosylation end products to collagen probably is irreversible.28,29
Skin changes
Skin thickness in the patient’s hands is assessed by attempting to tent the skin on the dorsum of the fingers between the examiner’s thumb and index finger. When skin changes are severe, loss of the transverse digital skin ridges on the dorsum of the fingers is obvious.
Seibold1 found skin changes in 34% of children with DM compared with no skin changes in healthy children; 20% of the patients with DM had involvement limited to the PIP joints and distally, 10% had changes extending to the MCP joints, and only 4% had adherent skin proximal to the MCP joints. Flexion contractures of LJM were found in the children who hmainlyad more extensive and severe skin changes. Seibold concluded that the digital flexion contractures are the result of thickening of the periarticular skin and subcutaneous tissue.
However, these skin changes also have been found in patients with DM who did not have LJM (and have been referred to as “diabetic sclerodactyly”). Being able to distinguish between diabetic sclerodactyly and scleroderma diabeticorum-pitting edema of the skin of the trunk and neck in some patients who have type 2 DM-is important.
Limited biopsy studies have revealed thickening of the dermis along with accumulation of connective tissue in the lower dermis and a paucity of glands and hair follicles in patients with DM.4,7,30 These skin changes have been likened to those seen in patients with scleroderma. Both DM and scleroderma manifest increased production of collagen by dermal fibroblasts in culture and can be strongly linked to abnormalities of the microvascular circulation.
Microangiopathy may eventually lead to similar secondary fibrotic changes in the affected skin in DM and scleroderma. However, the pathogenesis in LJM seems to be more related to metabolic than to immunological causes. HLA antigen histocompatibility typing done in 2 small groups has not indicated that patients with LJM form a distinct subgroup.26,31 In addition, Lieberman and coworkers32 showed that insulin pump treatment of patients with juvenile DM diminishes the skin thickness concurrently with a decrease in the levels of HbA1c, lending support to a metabolic rather than an immunological cause.
Association with
DM complications
In multiple studies, LJM has been found to correlate with the duration of DM.5,11,33 Campbell and colleagues2 found a general but asymptomatic reduction in joint mobility as early as 2 years after the diagnosis of DM, with continuing gradual deterioration as the duration of DM increased. Joint contractures were prevalent only in patients with long-standing DM (disease duration of 9 years or longer).
The researchers also found that limitation of joint mobility was more marked in patients with a prepubertal onset of DM than in those in whom the diagnosis was made after puberty. They explained this finding with the hypothesis that hyperglycemia results in the laying down of large amounts of highly glycosylated collagens during the pubertal growth spurt.34 The finding was confirmed by a longitudinal cohort study that reported an increased risk of LJM with longer duration of DM and with puberty.35
Rosenbloom and associates7 reported that after 16 years of juvenile DM, patients with LJM had a more than 3-fold greater risk of clinically apparent microvascular disease (retinopathy and nephropathy) than those who did not have LJM. Fitzcharles and coworkers36 found a similar but less dramatic association of microvascular disease with LJM in adult patients with DM.
Jung and colleagues37 reported an association of neuropathy with joint contractures in adult patients with DM by showing delayed median and ulnar nerve conditions and intrinsic hand muscle wasting in these patients. Results of subsequent cross-sectional studies differed and were inconclusive. Prospective data are scarce.
The authors of the Oxford Regional Prospective Study attempted to explore the temporal relationship between the development of LJM and microvascular complications.35 Although the albumin to creatinine ratio was higher in patients with LJM than in those without LJM, there was no difference between the groups in the prevalence of microalbuminuria. After disease onset, however, the presence of microalbuminuria was increased in patients with LJM. The authors concluded that the presence of LJM confers a 1.9-fold increased risk of this complication.
Arkkila and associates10 found a 3.1-fold higher risk of coronary heart disease and a 4-fold higher risk of cerebrovascular disease in patients who had type 2 DM with LJM. One study that analyzed the risk separately in men and women found that only in women were type 1 DM and LJM associated with subclinical macroangiopathy (greater carotid intima-medial thickness and higher risk of plaques). This association was not present in men with DM. However, men with DM and LJM were more likely to have proteinuria, retinopathy, and hypertension; in women with LJM, there was not an increased rate of these problems.38
Musculoskeletal and
other associations
Additional rheumatologic problems, such as shoulder bursitis, tendinitis, epicondylitis, osteoarthritis, and Dupuytren contracture, have been found to occur more frequently in patients with DM who have LJM than in those who do not.36 Fisher and coworkers39 reported an increased prevalence of frozen shoulder in patients with type 1 DM who had LJM and suggested that both conditions may be related to underlying abnormalities in glycosylation of collagen. LJM also has been associated with short stature4,5,35; insulin therapy36; poor DM control and higher HbA1c levels2,35; elevated plantar foot pressures, with increased risk of foot ulceration11; fibrous disease of the breast31; and restrictive lung disease.30,40
Ultrasonography
Ismail and colleagues41 reported a hypoechoic thickening of the flexor tendon sheaths in 12 of 14 patients who had DM with LJM, 3 of 17 unaffected patients with DM, and 2 of 10 healthy controls. Additional quantitative analyses revealed that patients with LJM had a tendon sheath thickness of more than 1 mm, compared with a thickness of less than 1 mm in unaffected patients with DM and controls.
Collier and associates42 used ultrasonography to study skin thickness in 92 patients with DM and 40 without. They found that thickness increases with the duration of DM and is closely related to the presence of LJM. Abate and colleagues12 used ultrasonography to evaluate supraspinatus, patellar, and Achilles tendons. There were significantly more sonographic “major lesions” or degenerative changes in the supraspinatus and Achilles tendons of patients with DM than in controls.
Treatment
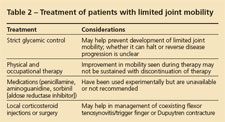
Treatment of patients with LJM remains largely unsatisfactory and controversial (Table 2). Strict control of blood glucose levels usually is advocated. Although numerous cross-sectional studies have not confirmed an association of LJM with glycemic control, as measured by HbA1c values, a few longitudinal studies have shown an increased risk of LJM in the presence of higher HbA1c levels.35,43
Two studies, one in children44 and the other in adults,45 demonstrated a reduced frequency of LJM compared with the frequency 2 decades ago. This change has been attributed to more intensive and aggressive glucose control strategies that have developed in the interim.
Although no large trials have demonstrated the effect of improved glycemic control on LJM, there is some anecdotal evidence. Case reports have demonstrated resolution of hand changes with improved control of DM.46 One patient with LJM began to notice improvement within a few weeks of pancreas transplant and had full range of motion in both hands at 6 weeks. Whether this improvement was a result of the improved glycemic control or the aggressive immunosuppression (including corticosteroids) that he received posttransplant remains controversial.47 Our own experience with a patient showed an improvement in his ability to press his palms together in the prayer sign, along with a decrease in his HbA1c values from 11.4% to 8.2%, during a 4-month period (see Figure 1).
Physical therapy with passive palmar stretching and forced progressive digital extension along with occupational therapy may improve function. A small pilot study in 11 patients found temporary but significant improvement in joint mobility after 10 sessions of passive joint mobilization at the rate of 2 sessions per week.48
In another study, injection of the palmar flexor tendon sheath with corticosteroids was found to improve contractures in patients with LJM.49 However, the results were difficult to interpret because coexistence of trigger finger was common in these patients.
Some drugs (eg, penicillamine and aminoguanidine) have been shown to prevent glycosylation of proteins and inhibit collagen cross-linking in experimental models of DM.25 However, they have considerable toxicities and are not recommended for routine clinical use.14 Improvement in LJM with the use of an aldose reductase inhibitor, sorbinil, was reported.16 However, these favorable results were not reproduced with use of another agent of the same class, ponalrestat.50 Aldose reductase inhibitors have not been marketed because of hepatotoxicity. Comorbid conditions (eg, Dupuytren contracture and flexor tenosynovitis/trigger finger) usually respond to local injection with corticosteroids or to surgical release.
References:
References
1. Seibold JR. Digital sclerosis in children with insulin-dependent diabetes mellitus. Arthritis Rheum. 1982;25:1357-1361.
2. Campbell RR, Hawkins SJ, Maddison PJ, Reckless JP. Limited joint mobility in diabetes mellitus. Ann Rheum Dis. 1985;44:93-97.
3. Lundbaek K. Stiff hands in long-term diabetes. Acta Med Scand. 1957;158:447-451.
4. Rosenbloom AL, Frias JL. Diabetes mellitus, short stature and joint stiffness-a new syndrome. Clin Res. 1974;22:92A.
5. Grgic A, Rosenbloom AL, Weber FT, et al. Joint contracture-common manifestation of childhood diabetes mellitus. J Pediatr. 1976;88(4 pt 1):584-588.
6. Benedetti A, Noacco C, Macor S, Pittaro I. Joint lesions in diabetes. N Engl J Med. 1975;292:1033-1034.
7. Rosenbloom AL, Silverstein JH, Lezotte DC, et al. Limited joint mobility in childhood diabetes mellitus indicates increased risk for microvascular disease. N Engl J Med. 1981;305:191-194.
8. Lawson PM, Maneschi F, Kohner EM. The relationship of hand abnormalities to diabetes and diabetic retinopathy. Diabetes Care. 1983;6:140-143.
9. Pal B, Anderson J, Dick WC, Griffiths ID. Limitation of joint mobility and shoulder capsulitis in insulin- and non-insulin-dependent diabetes mellitus. Br J Rheumatol. 1986;25:147-151.
10. Arkkila PE, Kantola IM, Viikari JS. Limited joint mobility in non-insulin-dependent diabetic (NIDDM) patients: correlation to control of diabetes, atherosclerotic vascular disease, and other diabetic complications. J Diabetes Complications. 1997;11:208-217.
11. Fernando DJ, Vernidharan J. Limited joint mobility in Sri Lankan patients with non-insulin-dependent diabetes. Br J Rheum. 1997;36:374-376.
12. Abate M, Schiavone C, Pelotti P, Salini V. Limited joint mobility (LJM) in elderly subjects with type II diabetes mellitus. Arch Gerontol Geriatr. 2010 Oct 10; [Epub ahead of print].
13. Larkin JG, Frier BM. Limited joint mobility and Dupuytren’s contracture in diabetic, hypertensive, and normal populations. Br Med J (Clin Res Ed). 1986;292:1494.
14. Kapoor A, Sibbitt WL Jr. Contractures in diabetes mellitus: the syndrome of limited joint mobility. Semin Arthritis Rheum. 1989;18:168-180.
15. Duffin AC, Donaghue KC, Potter M, et al. Limited joint mobility in the hands and feet of adolescents with Type 1 diabetes mellitus. Diabet Med. 1999;16:125-130.
16. Eaton RP, Sibbitt WL Jr, Harsh A. The effect of an aldose reductase inhibiting agent on limited joint mobility in diabetic patients. JAMA. 1985;253:1437-1440.
17. Pal B, Daly M. Skin-fold thickness and nail-fold capillaries in diabetics: relationship to limited joint mobility and retinopathy. Br J Rheumatol. 1987;26:153-155.
18. Trapp RG, Soler NG, Spencer-Green G. Nailfold capillaroscopy in type I diabetics with vasculopathy and limited joint mobility. J Rheumatol. 1986;13:917-920.
19. Sauseng S, Kästenbauer T, Irsigler K. Limited joint mobility in selected hand and foot joints in patients with type 1 diabetes mellitus: a methodology comparison. Diabetes Nutr Metab. 2002;15:1-6.
20. Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984;101:527-537.
21. Schnider SL, Kohn RR. Effects of age and diabetes mellitus on the solubility and nonenzymatic glycosylation of human skin collagen. J Clin Invest. 1981;67:1630-1635.
22. Seibold JR, Uitto J, Dorwart BB, Prockop DJ. Collagen synthesis and collagenase activity in dermal fibroblasts from patients with diabetes and digital sclerosis. J Lab Clin Med. 1985;105:664-667.
23. Bassiouny AR, Rosenberg H, McDonald TL. Glucosylated collagen is antigenic. Diabetes. 1983;32:1182-1184.
24. Kilo C, Vogler N, Williamson JR. Muscle capillary basement membrane changes related to aging and to diabetes mellitus. Diabetes. 1972;21:881-905.
25. Chang K, Uitto J, Rowold EA, et al. Increased collagen cross-linkages in experimental diabetes: reversal by beta-aminopropionitrile and D-penicillamine. Diabetes. 1980;29:778-781.
26. Gertner E, Sukenik S, Gladman DD, et al. HLA antigens and nailfold capillary microscopy studies in patients with insulin dependent and noninsulin dependent diabetes mellitus and limited joint mobility. J Rheumatol. 1990;17:1375-1379.
27. Gabbay KH. The sorbitol pathway and complications of diabetes. N Engl J Med. 1973;288:831-836.
28. Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315-1321.
29. Lyons TJ, Bailie KE, Dyer DG, et al. Decrease in skin collagen glycation with improved glycemic control in patients with insulin-dependent diabetes mellitus. J Clin Invest. 1991;87:1910-1915.
30. Buckingham BA, Uitto J, Sandborg C, et al. Scleroderma-like syndrome and the non-enzymatic glucosylation of collagen in children with poorly controlled insulin dependent diabetes (IDDM). Pediatr Res. 1981;15:626.
31. Soler NG, Khardori R. Fibrous disease of the breast, thyroiditis, and cheiroarthropathy in type I diabetes mellitus. Lancet. 1984;1:193-195.
32. Lieberman LS, Rosenbloom AL, Riley WJ, Silverstein JH. Reduced skin thickness with pump administration of insulin. N Engl J Med. 1980;303:940-941.
33. Garza-Elizondo MA, Diaz-Jouanen E, Franco-Casique JJ, Alarcón-Segovia D. Joint contractures and scleroderma-like skin changes in the hands of insulin-dependent juvenile diabetics. J Rheumatol. 1983;10:797-800.
34. Kohn RR, Schnider SL. Glucosylation of human collagen. Diabetes. 1982;31(suppl 3):47-51.
35. Amin R, Bahu TK, Widmer B, et al. Longitudinal relation between limited joint mobility, height, insulin-like growth factor 1 levels, and risk of developing microalbuminuria: the Oxford Regional Prospective Study. Arch Dis Child. 2005;90:1039-1044.
36. Fitzcharles MA, Duby S, Waddell RW, et al. Limitation of joint mobility (cheiroarthropathy) in adult noninsulin-dependent diabetic patients. Ann Rheum Dis. 1984;43:251-254.
37. Jung Y, Hohmann T, Gerneth JA, et al. Diabetic hand syndrome. Metabolism. 1971;20:1008-1015.
38. Frost D, Beischer W. Limited joint mobility in type 1 diabetic patients: associations with microangiopathy and subclinical microangiopathy are different in men and women. Diabetes Care. 2001;24:95-99.
39. Fisher L, Kurtz A, Shipley M. Association between cheiroarthropathy in frozen shoulder in patients with insulin-dependent diabetes mellitus. Br J Rheumatol. 1986;25:141-146.
40. Buckingham BA, Uitto J, Sandborg C, et al. Scleroderma-like changes in insulin-dependent diabetes mellitus: clinical and biochemical studies. Diabetes Care. 1984;7:163-169.
41. Ismail AA, Dasgupta B, Tanqueray AB, Hamblin JJ. Ultrasonographic features of diabetic cheiroarthropathy. Br J Rheumatol. 1996;35:676-679.
42. Collier A, Mathews DM, Kellett HA, et al. Change in skin thickness associated with cheiroarthropathy in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed). 1986;292:936.
43. Silverstein JH, Gordon G, Pollock BH, Rosenbloom AL. Long-term glycemic control influences the development of limited joint mobility in type 1 diabetes. J Pediatr. 1998;132:944-947.
44. Infante JR, Rosenbloom AL, Silverstein JH, et al. Changes in frequency and severity of limited joint mobility in children with type 1 diabetes mellitus between 1976-78 and 1998. J Pediatr. 2001;138:33-37.
45. Lindsay JR, Kenney L, Atkinson AB, et al. Reduced prevalence of limited joint mobility in type 1 diabetes in a U.K. clinic population over a 20-year period. Diabetes Care. 2005;28:658-661.
46. Lister DM, Graham-Brown RA, Burden AC. Resolution of diabetic cheiroarthropathy. Br Med J (Clin Res Ed). 1986;293:1537.
47. Hider SL, Roy DK, Augustine T, et al. Resolution of diabetic cheiroarthropathy after pancreatic transplantation. Diabetes Care. 2004;27:2279-2280.
48. Dijs HM, Roofthooft JMA, Driessens MF, et al. Effect of physical therapy on limited joint mobility in the diabetic foot. J Am Pod Med Assoc. 2000;90:126-132.
49. Sibbitt WL Jr, Eaton RP. Corticosteroid responsive tenosynovitis is a common pathway for limited joint mobility in the diabetic hand. J Rheumatol. 1997;24:931-936.
50. Rosenbloom AL, Buithieu M, Jelliffe KA, et al. Effect of an aldose reductase inhibiting agent on limited joint mobility in IDDM. Diabetes Care. 1992;15:588-589.




