Article
Making Sense of Hepatitis C Management in Chronic Kidney Disease
Author(s):
Kim Zuber, PA-C, and Jane Davis, DNP, provide perspective on guideline recommendations for the management of hepatitis C in people with chronic kidney disease from the Kidney Disease Improving Global Outcomes.
Jane Davis, DNP, and Kim Zuber, PA-C

Expert guidelines for evaluation, testing and management of hepatitis C (HCV) in chronic kidney disease (CKD) have gone through 2 major iterations in the last decade. The first discussion of how to treat the CKD patient was published in 2008.1 In 2018, the introduction of new oral direct acting antiviral (DAA) therapies necessitated a new guideline.2
However, Kidney Disease Improving Global Outcomes (KDIGO), the nephrology ‘gold standard’, has decided that an update is needed with new DAA protocols and the increasing use of HCV+ organ transplants.3 There is no update on who to screen nor how to screen, no update on transmission in dialysis units but there are changes in how to treat HCV in CKD (Chapter 2) and how to manage HCV in kidney transplant patients. (Chapter 4).3
KDIGO recommends treating all patients with CKD, Stage 1 through Stage 5D (on dialysis), with DAA therapy (Figure 1) Of course, as is standard for all HCV protocols, patients should be tested for hepatitis B prior to treatment.
Hepatitis C treatment Regimen by CKD Stage3
Source: Kidney Disease: Improving Global Outcomes (KDIGO) Hepatitis C Work Group. KDIGO 2022 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease
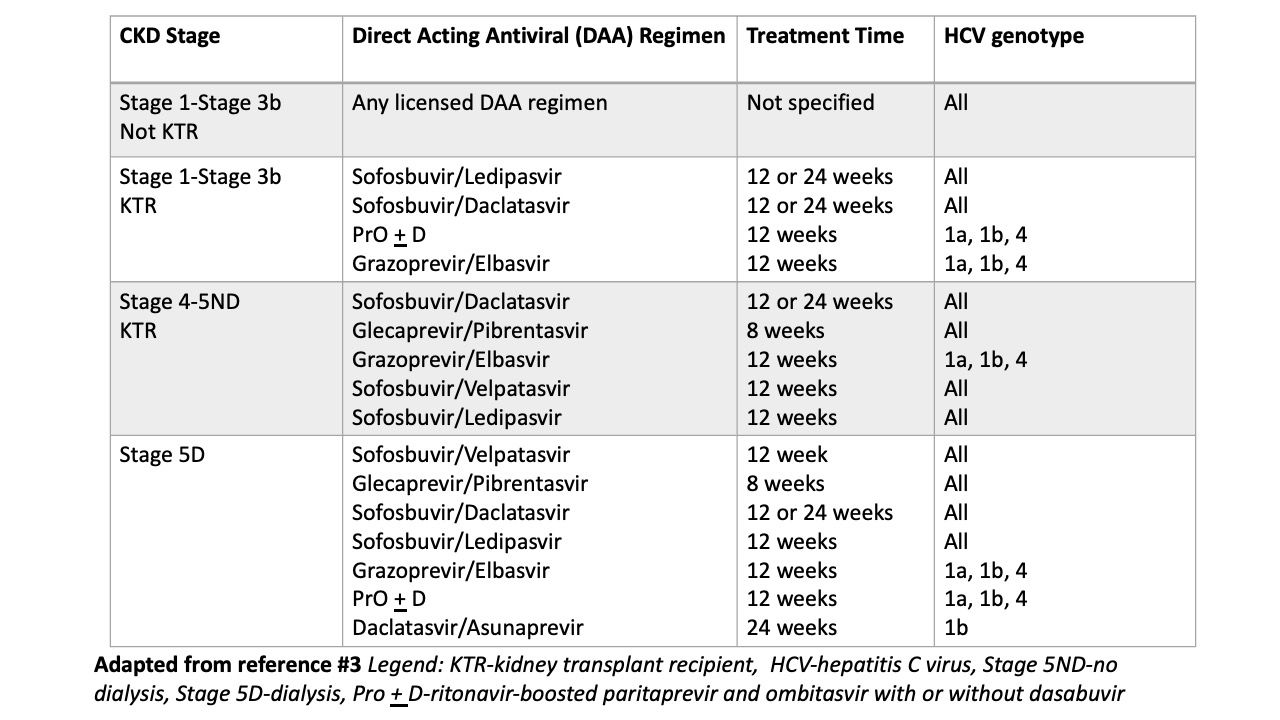
Treatment is condensed in the figure with the caveat that the order of treatments in the figure does not indicate a ranking or preferential order. Note is made that sofosbuvir is safe for all stages of CKD.3
The biggest change in the guidelines is in the kidney transplant patient. While there have been concerns of drug-drug interactions with the medications used for immunosuppression, recent trials have shown the DAA medications are safe in this population. We know that if left untreated, HCV will decrease patient survival after kidney transplant. For patients who are HCV+, the timing of treatment depends on the transplant plan and the level of liver fibrosis (high liver fibrosis means a liver/kidney transplant is recommended, rather than kidney only). If the HCV+ patient has a living donor and is expected to be transplanted quickly (defined as less than 24 weeks), then the recommendation is to treat with DAA therapy after the transplant. If the wait time for the living donor is less than 24 weeks, the recommendation is to treat as soon as possible with a DAA.3
If the patient does not have a living donor and a deceased donor HCV+ kidney is used, then treatment is after transplant. Transplant with HCV+ kidneys is encouraged by KDIGO due to the excellent outcomes with these organs. Treatment for HCV+ should occur as soon as possible post-transplant with DAA therapy. However, due to the more frequent relapse rate, treatment should be for the full 12 weeks no matter which protocol is used.3
KDIGO’s new guidelines for the treatment of CKD patients place a special emphasis on the kidney transplant patient. As more and more of our patients are transplanted with HCV+ organs, experience with DAA regimens has shown them to be a safe and effective treatment in all stages of CKD. Dialysis is a bridge to transplant for patients able to qualify and tolerate transplant surgery. Survival on dialysis is limited and survival with a transplant is statistically much better. HCV+ organs will allow better long-term survival in kidney failure patients and the use of these organs is encouraged by the entire nephrology community.
References:
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008 Apr;(109):S1-99.
- Gordon CE, Balk EM, Francis JM. Summary of the 2018 Kidney Disease Improving Global Outcomes (KDIGO) Guideline on hepatitis C in chronic kidney disease. Semin Dial. 2019 Mar;32(2):187-195.
- Kidney Disease: Improving Global Outcomes (KDIGO) Hepatitis C Work Group. KDIGO 2022 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int. 2022;102(6S):S129–S205.




