Article
Managing fibromyalgia: An update on diagnosis and treatment
Fibromyalgia syndrome (FMS) is characterized by widespread chronic pain and tenderness. Persons with FMS are a diverse population, with widely variable symptom presentation and severity, as well as secondary symptoms. Because the symptoms are so diverse, diagnosis and management become challenging. Mounting evidence supports altered CNS processing of nociceptive stimuli as a mechanism.
ABSTRACT: Fibromyalgia syndrome (FMS) is characterized by widespread chronic pain and tenderness. Persons with FMS are a diverse population, with widely variable symptom presentation and severity, as well as secondary symptoms. Because the symptoms are so diverse, diagnosis and management become challenging. Mounting evidence supports altered CNS processing of nociceptive stimuli as a mechanism. American College of Rheumatology criteria have become accepted as the standard classification system for clinical research, but their use is controversial in the clinical setting. Diagnosis is based on a combination of the patient history, reports of clinical and evoked pain, and laboratory testing. Treatment currently focuses on controlling pain, improving sleep, reducing affective distress, and increasing physical function with a combination of pharmacological and nonpharmacological therapies.
Fibromyalgia syndrome (FMS) is characterized by widespread chronic pain and tenderness at specific points across the body. The syndrome affects about 2% to 3% of the general US population and 4% of the female population.1 The ratio of female to male treatment-seekers ranges from 7 to 9 to 1.2 FMS has been observed in children, but its prevalence tends to peak in the fourth to sixth decade of life.
The classic patient who has FMS presents with pain at multiple locations throughout her body, reports severe fatigue and stiffness, and describes difficulty in obtaining adequate sleep. However, persons with FMS are a diverse population, with widely variable symptom presentation and severity. Presentation of secondary symptoms, including cognitive dysfunction, abdominal pain, and headaches, is common. Because the symptoms reported are so diverse, diagnosis and management become challenging.
In the medical literature, the set of symptoms associated with FMS has appeared in various forms and with different names (eg, fibrositis, tension myalgia, psychogenic rheumatism) since the early 1900s. In addition to the problem of multiple labels, diverse criteria (eg, pain reported on palpation of 11, 22, or 43 locations) and symptoms (eg, sleep disorders, diarrhea, headaches, fatigue) have been presented as core features of FMS. Given the inconsistencies in the literature, some authors have suggested that FMS is merely a vague set of associated symptoms that does not warrant a specific label and, furthermore, any label such as FMS is detrimental to the patient because it supports the person's belief that he or she is ill.
The complexity of symptom presentation in FMS contributed to a 3-fold increase in total health care costs in patients with the condition compared with a control group of patients randomly selected from an insurance database.3 Given the prevalence and increased health care utilization, primary care physicians encounter patients with FMS on a routine basis and have a special need for current information with regard to diagnosis and treatment recommendations.
In this article, we highlight recent developments about the diagnosis of FMS and evidence-based clinical practice guidelines for treatment. We outline current knowledge of the mechanisms that may underlie FMS, review current recommendations for disease classification, provide a diagnostic algorithm that may be used in clinical assessment, present both pharmacological and nonpharmacological treatment options, and describe American Pain Society (APS) treatment recommendations.
CAUSES AND PATHOPHYSIOLOGY
The causes and pathophysiology of FMS remain unclear; however, growth in understanding of the mechanisms has been seen over the past decade. It was thought originally that there was a peripheral mechanism involving the musculoskeletal system that manifested with reports of pain after palpation of specific locations-"tender points." Now it is more widely accepted that patients with FMS are generally more tender "all over," meeting the criterion of "widespread pain," and experience a generalized increased sensitivity to sensory stimuli.4
Consistent with this idea, mounting evidence supports altered CNS processing of nociceptive stimuli as a mechanism.5 A number of biochemical abnormalities consistent with CNS dysregulation have been demonstrated, including lower serum serotonin and norepinephrine levels and higher substance P and nerve growth factor levels in cerebrospinal fluid. Psychophysiological studies also demonstrate enhanced temporal summation of pain and altered cerebral blood flow among brain regions involved in the processing of pain. These altered CNS mechanisms also have been identified in patients with other functional syndromes, such as irritable bowel syndrome, temporomandibular disorders, and chronic headache, which are common comorbidities in the FMS population.
The factors contributing to this central sensitivity remain unknown; however, multiple factors have been implicated, including genetic predisposition, endocrine dysfunction, physical and psychological trauma, illness, and various psychosocial factors.6 Although the trend has been to view FMS as a CNS disorder, a recent study demonstrated that peripheral input also may play a key role in maintaining central sensitization.7
In spite of advances in understanding of the theoretical construct of FMS, the practice of making the diagnosis remains unchanged. There currently is no cure for FMS, and the focus of treatment continues to be on symptom management. Although a large number of both pharmacological and nonpharmacological treatments have been developed and evaluated, many patients do not receive adequate symptom relief to improve functional limitations.
DIAGNOSISHistory of nomenclature and classification
FMS has been formally recognized for more than 15 years, is thought to be a prevalent disorder, and has an extensive literature, but there is no gold standard for the diagnosis. Without a clear biological marker for the disorder, diagnosis is based exclusively on patients' self-report and ruling out of other diseases that have symptoms in common.
In 1990, the American College of Rheumatology (ACR) proposed a classification system for FMS composed of 2 specific criteria: (1) chronic widespread pain and (2) report of pain on palpation (4 kg of pressure) at 11 or more of 18 specific tender points.8 In the differentiation of patients with FMS from those with low back pain or rheumatoid arthritis, the ACR criteria had a sensitivity of 88.4% and specificity of 81.1%.8 Therefore, they appeared to offer an acceptable level of accuracy in diagnosis and, more important, a consistent set of classification criteria to be used in research.
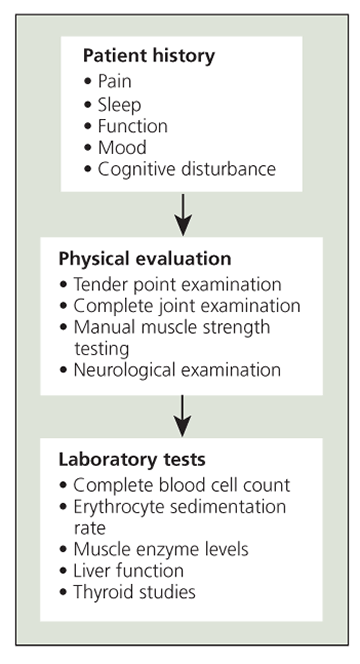
Figure 1 – A standard diagnostic protocol for fibromyalgia syndrome is shown in this diagram.
The ACR criteria have become accepted as the standard classification system for FMS clinical research. However, their use is controversial in the clinical setting and they are not applied consistently for patient diagnosis. Clinically, diagnosis is based on a combination of the patient history, reports of clinical and evoked pain, and laboratory testing to assess comorbid or differential diagnoses. Figures 1 and 2 show standard diagnostic practices for FMS and a diagnostic algorithm recommended by the APS, respectively.9
Current clinical diagnostic recommendationsHistory. Diagnosis begins by taking a careful history of the patient's current level of pain, sleep, and function. In addition to the core set of symptoms that persons with FMS report-chronic widespread pain and fatigue-they also may describe anxiety, depression, GI symptoms, headaches, and difficulty in concentrating. Physicians should pay careful attention in the history assessment for signs of psychosocial distress, because patients with distress may experience greater functional impairment and require closer follow-up and more in-depth therapy.
Figure 2 – A diagnostic algorithm for fibrom
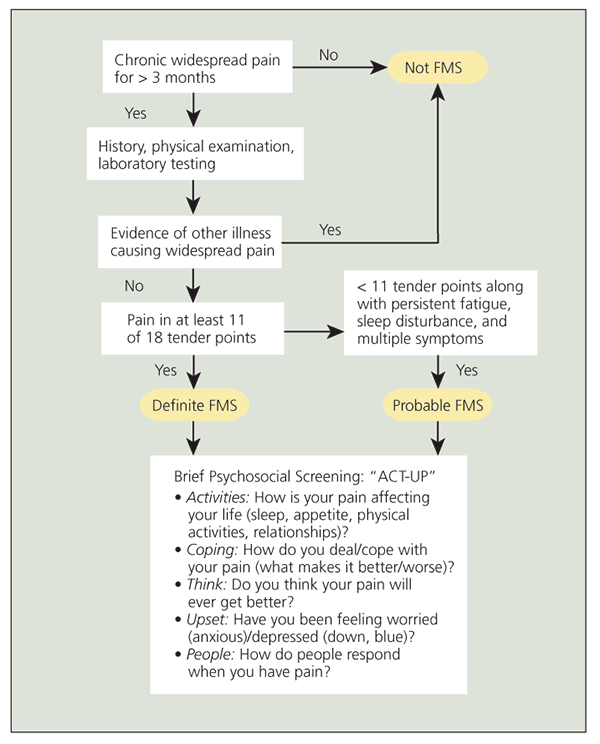
yalgia syndrome (FMS) recommended by the American Pain Society is seen in this flowchart.
Physical examination.
The history may be followed with a tender point examination. In practice, fewer than 11 tender points may be accepted if other supportive clinical features (eg, fatigue, morning stiffness, sleep difficulty) are present. In addition to the tender point examination, physicians should perform general musculoskeletal and neurological examinations. Depending on the results of these examinations, additional laboratory tests, imaging studies, and electrodiagnostic evaluations might be considered.9
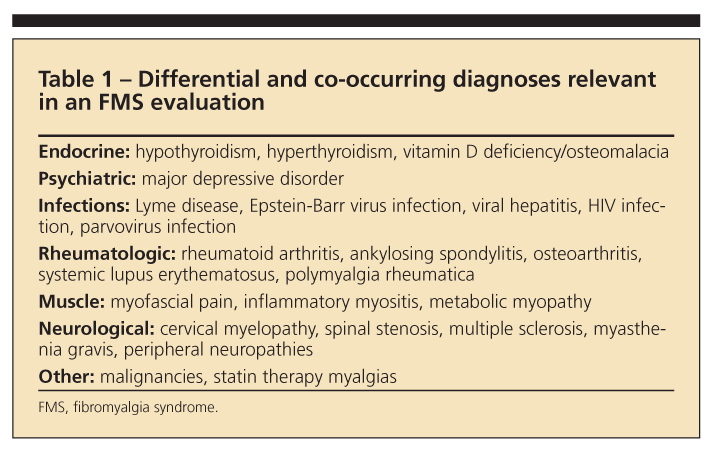
Laboratory tests. There currently is no diagnostic test for FMS. Common causes of chronic widespread pain that may be difficult to differentiate from FMS or that may co-occur are listed in Table 1. Tests recommended by the APS include complete blood cell count, erythrocyte sedimentation rate, thyroid studies, and chemistry panel.
Recent criticisms about diagnostic practice
In spite of published standards for the diagnosis of FMS,9 diagnostic practices are highly variable, contributing to a heterogeneous population of
persons with FMS. The lack of consistency in diagnostic practice arguably is the greatest criticism about FMS diagnosis.
Fitzcharles and Boulos10 evaluated patients newly referred for rheumatology consultation. Of 76 patients who were referred with a final or questionable FMS diagnosis, only 26 (34%) met the ACR diagnostic criteria. In a paper that compared the overlap between clinical and ACR criteria, Katz and colleagues11 reported that 49% of patients evaluated met the clinical criteria but only 29.1% met the ACR criteria.
Clearly, the ACR criteria are more stringent than what is implemented clinically. Although Fitzcharles and Boulos interpreted their findings by suggesting that FMS is overdiagnosed, others have suggested that FMS exists on a continuum and is underdiagnosed when the ACR criteria are implemented. Because including only patients who meet the ACR criteria in clinical trials is standard practice, results from trials may not generalize to the broader FMS population. Considering this discrepancy between the definitions of clinical and empirical FMS is important when clinical trials are evaluated.
The variability in diagnostic practices stems, in part, from disagreement over the role of tender points. It was thought historically that FMS is a soft tissue disorder with multiple areas of localized tenderness and that there is something pathologically wrong with the specific tender point sites. Current understanding suggests that patients with FMS are more sensitive to both painful and nonpainful sensory stimuli as a result of CNS dysregulation. If the latter is the case, tender points may serve as a marker for a generalized increased sensitivity and fewer tender points may be needed for diagnosis.
Another criticism of the ACR criteria is that with a focus on the widespread pain and tender point criteria, the complex symptom experience among patients with FMS is not captured. In particular, the psychosocial and distress elements of the disorder become largely ignored. Yunus12 emphasized the importance of the symptom experience in the diagnosis of FMS before and during the development of the ACR criteria in 1990. Some authors have suggested more recently that diagnoses should rely less on tender point criteria and more on secondary symptoms.13
Alternatives to the ACR criteria have been suggested. Some researchers recommend that fewer tender points are needed to make an accurate diagnosis. Harden and colleagues14 compared active and control tender point sites between 25 patients with FMS and a convenience sample of 31 healthy controls. They found not only that the groups differed on both control and active sites but also that the control sites could be used for diagnosis; the overall classification accuracy was 85.7%.
In the Katz and colleagues11 article, the survey criteria were moderately concordant with the ACR criteria (75%) and the authors suggested that they may be used as an alternative to a clinical examination. Wolfe, the lead researcher involved in the development of the ACR criteria, has suggested that by focusing on the total number of tender points and evaluations of fatigue, a more complete representation of the FMS population may be realized.
TREATMENT
Until the causes and mechanisms of FMS are well defined, the development of a cure for FMS is unlikely. Treatment currently focuses on controlling pain, improving sleep, reducing affective distress, and increasing physical function with some combination of pharmacological and nonpharmacological therapies.
Clinicians should start treatment by confirming the diagnosis, educating the patient about FMS and, with the patient, developing realistic treatment goals. The diagnosis may be made and treatment may be successful in the primary care setting with a combination of pharmacological and nonpharmacological therapies. However, patients who have persistent symptoms, significantly reduced levels of physical functioning, and elevated levels of emotional distress should be referred to a mental health professional for cognitive-behavioral therapy (CBT) and to a physical therapist for an individualized exercise plan.9 Patients who have multiple comorbidities and complex symptom patterns may benefit from referral to an interdisciplinary pain rehabilitation facility that addresses the medical, physical, and emotional components of FMS and related disabilities.
Pharmacological
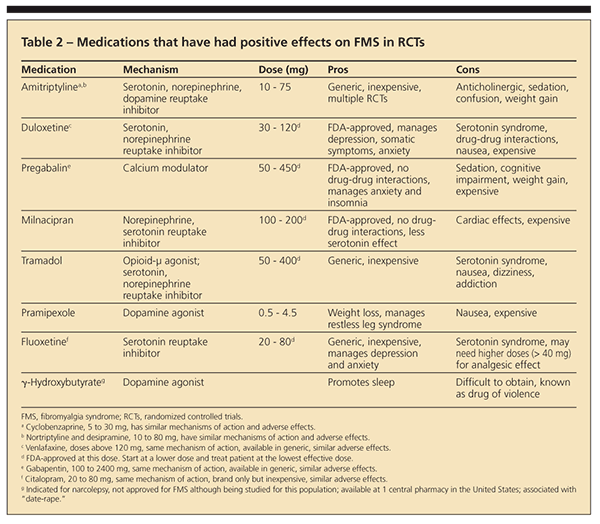
Numerous trials have investigated the effectiveness of a large number of pharmaceutical agents. Several studies have reported statistically significant results, but as do most chronic pain treatments, drugs offer a modest effect at best, averaging 34% improvement. 15Table 2 presents pharmacological agents that have received modest support in the management of FMS; dosages, advantages, and disadvantages are included.
Tricyclic antidepressants (TCAs). TCAs, such as amitriptyline and cyclobenzaprine, work primarily by directly blocking the reuptake of serotonin and norepinephrine, thereby increasing the concentration of these neurotransmitters. Although evidence for the efficacy of this class of drugs for FMS is good,15 tolerability often is an issue. Therefore, starting these drugs at very low doses and increasing the dose very slowly is recommended. Because of toxicity, dispensing these drugs in a small number of tablets at any one time is recommended to prevent accidental or intentional death.
Selective serotonin reuptake inhibitors (SSRIs). SSRIs were developed to more specifically target serotonin in an effort to decrease adverse effects associated with the more broadly acting TCAs. The results of trials evaluating the efficacy of these more highly selective serotonin drugs, including fluoxetine and citalopram, are less consistent than the results of those with dual effects on norepinephrine and serotonin.16
Selective serotonin-norepinephrine reuptake inhibitors (SSNRIs). Given the inferior performance of the more selective serotonin acting drugs, more recent trials have begun focusing on the new SSNRIs. This class of drugs targets both serotonin and norepinephrine, but the agents do not interact with adrenergic, cholinergic, or sodium channels in the way TCAs do, thereby avoiding some of the adverse effect and tolerability issues. The evidence seen thus far suggests that duloxetine16 and milnacipran,17 both SSNRIs, are well tolerated and are effective in reducing the functional impact of having FMS. Both drugs have FDA approval.
Anticonvulsants. Gabapentin, used primarily for neuropathic pain conditions, has demonstrated efficacy for improving impaired function in patients with FMS.18 Another new treatment involves the use of the anticonvulsant pregabalin, which works by binding to the a2d subunit of the voltage-gated calcium channels. Calcium triggers the release of various neurotransmitters thought to play a role in chronic pain. By decreasing the calcium influx at nerve terminals, the drug decreases the amount of these neurotransmitters released. Patients who received pregabalin showed significant improvement in mean daily pain, sleep quality, and function compared with a placebo group.19 The drug has been approved by the FDA.
Pharmaceuticals not recommended. Although opioid analgesics and NSAIDs are prescribed frequently for patients with FMS, empirical support for this use is inadequate. The potential for abuse, in particular, has been noted as an impediment, and the APS guideline specifically recommends against their use. However, persons with FMS indicated in a large Web-based survey that opioids provided better pain relief than the FDA-approved drugs for FMS.20
Nonpharmacological
Psychosocial factors appear to play a key role in the maintenance and, possibly, the cause of FMS, and treatments aimed at improving coping skills and self-efficacy and decreasing negative thinking are equally useful for achieving successful outcomes. Many patients with FMS report that they are inactive because of high levels of fatigue and pain associated with physical activity. As a result, many are physically deconditioned, which may contribute to even greater disability.
Education about FMS, along with information about what to expect from treatment and the importance of self-management, is essential to effective FMS management.9 Although a large number of nonph
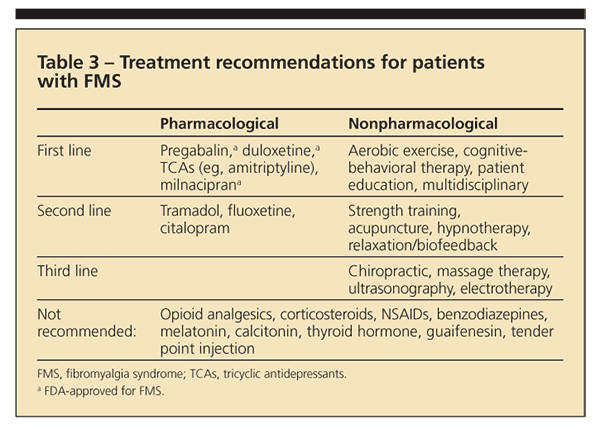
armacological treatments have been investigated and used in treatment, exercise and CBT have received the most attention and empirical support.
Exercise. The APS recommends exercise as a first-line treatment (Table 3). However, studies suggest that adherence to exercise recommendations is quite low and that barriers to exercise-including pain severity, fatigue, and depression-be addressed before and concurrent with exercise therapy.16
Cognitive-behavioral therapy. CBT is another APS-recommended first-line treatment. The major goals are to help patients understand the effects that thoughts, beliefs, and expectations have on their symptoms. Self-management is a central feature.
Multicomponent treatment
No single intervention has been demonstrated to be highly effective for the majority of patients with FMS. However, reasonably good evidence indicates that multicomponent, self-mastery approaches that include education, exercise, and CBT, often delivered to groups of patients by a multidisciplinary team, are helpful for many.9 Preliminary studies have supported the combination of pharmacological and nonpharmacological approaches to treatment.21
CONCLUSIONS
The best treatment appears to be a combination of education, pharmacological agents, physical therapy, and CBT. Communicating to patients that they need to accept responsibility for self-management in collaboration with their primary care physician is key. Physicians need to recognize the complexity and range of symptoms associated with FMS and expect treatment to be similar to that for other patients with chronic diseases, such as diabetes mellitus. Because patients with FMS often feel that they and their symptoms and distress are treated dismissively, support and encouragement from physicians is essential.
References:
References1. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, part II. Arthritis Rheum. 2008;58:26-35.
2. White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol. 1999;26:1577-1585.
3. Berger A, Dukes E, Martin S, et al. Characteristics and healthcare costs of patients with fibromyalgia syndrome. Int J Clin Pract. 2007;61:1498-1508.
4. Geisser ME, Strader Donnell C, Petzke F, et al. Comorbid somatic symptoms and functional status in patients with fibromyalgia and chronic fatigue syndrome: sensory amplification as a common mechanism. Psychosomatics. 2008;49:235-242.
5. Martinez-Lavin M. Biology and therapy of fibromyalgia: stress, the stress response system, and fibromyalgia. Arthritis Res Ther. 2007;9:216.
6. Okifuji A, Turk DC. Stress and psychophysiological dysregulation in patients with fibromyalgia syndrome. Appl Psychophysiol Biofeedback. 2002;27:129-141.
7. Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain. 2009;145:96-104.
8. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160-172.
9. Burckhardt C, Goldenberg D, Crofford L. Guideline for the Management of Fibromyalgia Syndrome Pain in Adults and Children. Glenview, IL: American Pain Society; 2005. APS Clinical Practice Guidelines Series 4.
10. Fitzcharles MA, Boulos P. Inaccuracy in the diagnosis of fibromyalgia syndrome: analysis of referrals. Rheumatology (Oxford). 2003;42:263-267.
11. Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis: a comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum. 2006;54:169-176.
12. Yunus MB. Diagnosis, etiology, and management of fibromyalgia syndrome: an update. Compr Ther. 1988;14:8-20.
13. Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatol. 2003;30:369-378.
14. Harden RN, Revivo G, Song S, et al. A critical analysis of the tender points in fibromyalgia. Pain Med. 2007;8:147-156.
15. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia: a meta-analysis and review. Psychosomatics. 2000;41:104-113.
16. Arnold LM. Biology and therapy of fibromyalgia: new therapies in fibromyalgia. Arthritis Res Ther. 2006;8:212.
17. Gendreau RM, Thorn MD, Gendreau JF, et al. Efficacy of milnacipran in patients with fibromyalgia. J Rheumatol. 2005;32:1975-1985.
18. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56:1336-1344.
19. Arnold LM, Russell IJ, Diri EW, et al. A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain. 2008;9:792-805.
20. Bennett RM, Jones J, Turk DC, et al. An internet survey of 2,596 people with fibromyalgia. BMC Musculo skelet Disord. 2007;8:27.
21. Fors EA, Sexton H, Götestam KG. The effect of guided imagery and amitriptyline on daily fibromyalgia pain: a prospective, randomized, controlled trial. J Psychiatr Res. 2002;36:179-187.




