Article
Managing Knee Osteoarthritis: Rationale for Early Treatment
Attention to early diagnosis and intervention in osteoarthritis (OA) has increased, but which treatments may have the greatest benefit is unclear. However, available and emerging treatments can ameliorate progression.
ABSTRACT: Attention to early diagnosis and intervention in patients with osteoarthritis (OA) has increased, but which treatments may have the greatest benefit in slowing or potentially reversing disease progression is unclear. Many interventions have significant positive effects on biomarkers potentially related to disease progression. In many patients, cartilage lesions are likely to be progressive; early intervention aimed at limiting damage to articular cartilage may be critical for decreasing chronic pain and disability. Current therapeutic approaches are considered primarily symptomatic, but there is potential to use available and emerging treatments to ameliorate the progression of OA. Needed are reliable biological markers. In the future, the focus will be not only on reducing pain and improving function but also on early detection and early intervention with chondroprotection.
Osteoarthritis (OA) has been estimated to affect about one-third of persons in the United States aged 65 years or older. Providing effective intervention aimed at avoiding long-term disability stemming from this disease is difficult because there is little capacity to heal articular cartilage damage and damage is likely to be progressive.
Although attention to early diagnosis and intervention in patients with OA has increased, which of the many currently available OA treatments are likely to have the greatest benefit in slowing or potentially reversing disease progression is unclear. There has been no consistent evidence that any treatment can slow the radiological progression of OA, but many interventions have been shown to have significant positive effects on biomarkers potentially related to disease progression.
This is the first article in a 3-part series on managing early knee OA in which we review clinical results for nonpharmacological and pharmacological treatments for patients with OA and summarize data related to their potential for altering disease progression. Although such information is limited, it may help clinicians select treatments for patients who have early OA.
In this first part, we describe the relationships among molecular changes, structural damage, and disease progression, as well as the rationale for early treatment and defining disease modification. The second article will discuss self-help and nonpharmacological interventions. In the third article, we will provide an overview of systemic pharmacotherapy, including acetaminophen, NSAIDs/cyclooxygenase 2 inhibitors, licofelone, diacerein, tramadol and other opioid analgesics, and calcitonin, as well as intra-articular treatments, including corticosteroids and hyaluronates.
METHODOLOGY
Searches of studies for all therapies were carried out using PubMed. Searches for each treatment were focused on citations that included information on “disease modification,” “biomarkers,” “joint space,” “inflammation,” and “cytokines.”
BACKGROUND
FIGURE
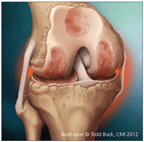
Osteoarthritis (OA) is characterized by structural changes, including progressive loss of articular cartilage, increased subchondral plate thickness, the formation of new bone at the joint margins (osteophytes), and the development of subchondral bone cysts.
OA is characterized by structural changes, including progressive loss of articular cartilage, increased subchondral plate thickness, the formation of new bone at the joint margins (osteophytes), and the development of subchondral bone cysts (Figure).1,2 Many factors that contribute to OA progression have been identified, and much of it may be mediated by aberrant biomechanical forces and pathological responses to them.3,4
Chondrocytes normally respond to mechanical stress by synthesizing extracellular matrix, including the primary components of the articular cartilage matrix, aggrecan, and type II collagen.5,6 However, abnormal forces in joints affected by OA alter chondrocyte metabolism. The changes result in decreased collagen synthesis and increased production of cartilage-degrading proteases, including matrix metalloproteinases (MMPs) and aggrecanases. Excessive cleavage of type II collagen in OA is thought to result from up-regulation of the synthesis and activity of collagenases, particularly MMP-13.
Chondrocytes in joints affected by OA change phenotype and resemble differentiating chondrocytes in endochondral ossification.5 These cells proliferate; express differentiation-related genes (eg, parathyroid hormone–related peptide); and produce increased levels of type X collagen, annexins, alkaline phosphatase, and osteocalcin.
Changes in Subchondral Bone
Cartilage degradation may be preceded by changes in subchondral bone, according to the results of studies of experimental animal models and patients with OA.7 Thickening of the subchondral bone plate may contribute to increased chondrocyte stress and damage to the overlying cartilage. Subchondral bone also may release catabolic factors that promote abnormal cartilage metabolism.8,9
Other changes in chondrocyte metabolism associated with OA include increased production of inflammatory mediators, such as interleukin (IL)-1β, IL-8, IL-6, tumor necrosis factor alpha α (TNF-α), prostaglandin E2, and nitric oxide. Proinflammatory cytokines, such as IL-1β and TNF-α, can promote cartilage resorption as well as drive recruitment and activation of other inflammatory cells. Of note, OA has many characteristics of systemic inflammatory disease and involves T-cell–mediated immune responses associated with increased levels of IL-1β and TNF-α.5,6 Synovial inflammation also is characteristic of OA, and cartilage degradation products are thought to stimulate synovitis in OA.7
Joint Tissue Composition Altered
In addition to declining levels of cartilage during OA, the composition of the joint tissue is altered. Histological evaluation of the knees in patients with OA has demonstrated synovial hypertrophy and hyperplasia, with an increase in the number of lining cells. Calcification of the extracellular matrix, a reduced level of proteoglycans, and an increase in apoptotic cells compared with normal cartilage also are evident by histological analysis.10 Intrusion of calcified cartilage into the articular cartilage may modify the contours of adjacent articulating surfaces within the joint.1,2
OA has been shown to involve vascular pathology and aberrant vascular invasion. Increased vascularization and abnormal innervation of articular cartilage may contribute to the development of tibiofemoral pain in patients with knee OA.8,11
Another possible marker for progression of OA is lubricin (superficial zone protein, proteoglycan 4), a mucinous glycoprotein secreted from synovial fibroblasts and superficial zone articular chondrocytes.12 It has been shown that levels of lubricating protein in cartilage are higher in patients with OA than in normal controls and that the concentration rises with increasing OA severity.13 However, this apparent compensatory response is not sufficient to reduce friction in knees with OA.
The elasticity of collagen is decreased in the cartilage of patients with knee OA.14 The concentration and viscosity of synovial fluid also are decreased.15
In a study that involved rabbits with sustained anterior cruciate ligament (ACL) and posterior cruciate ligament damage, the injury decreased lubricin levels and the reduction was associated with damage to the articular cartilage matrix.16 In a study of 30 patients with unilateral ACL insufficiency, lubricin concentrations in synovial fluid were reduced significantly at short intervals after ACL injury compared with those in the intact knee.12 Within 12 months, however, lubricin levels in the damaged knee approached those in the intact knee. In this study, TNF-α levels in injured knees were inversely correlated with lubricin levels.
RELATIONSHIPS WITH SYMPTOMS PROGRESSION
Structural Damage and Symptom Severity
Several studies have demonstrated close relationships between structural or molecular changes and the progression of symptom severity in patients with knee OA. In the Multicenter Osteoarthritis Study, which assessed 2210 persons at high risk for OA, 14% of them had stable radiographic OA and 27% had worsening radiographic OA between enrollment and the 30-month study end point.17 The risk of incident severe functional limitation in patients with worsening radiographic OA was more than double the risk in those with radiologically stable disease.
Molecular Changes and Structural Damage
In a study of 2483 patients that correlated early changes in biochemical markers of bone (cross linked N-telopeptide of type I collagen), cartilage (C-telopeptide of type II collagen [CTX-II]) degradation, and x-ray film results, a significant relationship was found between higher CTX-II levels and radiological disease progression.18 Also, MRI showed elevated CTX-II levels to be associated with decreases in the mean thickness of medial and lateral tibial cartilage over 1 year of follow-up.19
Assessment of concentrations of serum cartilage oligomeric matrix protein (COMP) and cleaved collagen neoepitope compared with cartilage volume in 4 compartments of the tibiofemoral joint showed a significant negative correlation between serum COMP and medial tibial cartilage volume in male patients with knee OA.20,21 In a small-scale study of 120 patients with knee OA and 45 normal controls, P selectin levels in synovial fluid were significantly correlated with OA severity, as reflected by Kellgren-Lawrence grade.22 Overexpression of bone morphogenetic protein-7 and levels of osteopontin and endoglin in both plasma and synovial fluid also have been shown to be correlated with progression of knee OA, as determined by Kellgren-Lawrence grading.23-25
RATIONALE FOR EARLY TREATMENT
Damaged articular cartilage has little or no healing capacity because its low metabolic activity and limited blood supply permit only a modest response to injury. In many patients, cartilage lesions are likely to be progressive.26 Therefore, early intervention aimed at limiting damage to articular cartilage may be critical for decreasing the chronic pain and disability associated with OA.
The cost of managing OA increases as the disease progresses. The annual cost of OA in the United States has been estimated at $128 billion27; a major component of the direct costs is the management of later-stage disease (eg, joint replacement).28 The indirect costs of OA also are elevated in patients with later-stage disease; in a 2010 study, annual costs resulting from lost productivity were $6096, $13,251, and $17,214 per patient for self-reported mild, moderate, and severe OA, respectively.29 Quality of life for patients with OA also declines with progression of the disease.30
Successful treatment for patients with early OA would greatly benefit them as well as the health care system because it could reduce long-term morbidity and the consequent high level of use of health service resources.26 Early diagnosis and management of OA (perhaps even before it becomes symptomatic) to prevent subsequent pain and disability associated with this disease is supported by the National Public Health Agenda for Osteoarthritis, published by the CDC and the Arthritis Foundation.31
DEFINING DISEASE MODIFICATION IN OA
A disease-modifying agent for OA has been defined as one that can slow, halt, or reverse the rate of disease progression. Of note, there is no current clear consensus on what end point in a clinical trial would provide evidence of disease-modifying capacity for an OA therapy.32 Although the current therapeutic approaches for OA are considered to be primarily symptomatic, there is potential to use available and emerging treatments to ameliorate the progression of OA.33
Detecting decreases in joint-space width on plain x-ray films is the currently recognized technique for evaluation of cartilage damage and OA structural progression.34-36 However, the use of plain x-ray films for assessment of disease progression in patients with knee OA has significant limitations. Most important, radiographic evidence of OA is seen only after significant cartilage degradation has already taken place.35 In addition, there are substantial differences in the apparent rate of joint-space width decline among knee OA patient cohorts that may be the result, at least in part, of inconsistent positioning of the knee when serial x-ray films are obtained and other technical factors.37
Reliable Biological Markers Needed
Needed are reliable biological markers that have the capacity to predict cartilage loss and that can be used for assessment of the disease-modifying effects of OA treatments. There is no generally accepted preradiographic biomarker that can be used to assess the potential disease-modifying effects. However, several candidate markers may be useful for assessing the potentially disease-modifying effects of therapy in the later disease stages.35,38 These include serum hyaluronic acid; serum COMP; urinary CTX-II; and serum and urinary Coll2-1 and its nitrated form, Coll2-1NO2.
The OA Biomarker Global Initiative, aimed at developing biomarkers for assessment of patients with OA, is a partnership with the National Institute on Musculoskeletal Diseases, the Osteoarthritis Research Society International, the American Orthopedic Society for Sports Medicine, and the Orthopaedic Research Society.39 The efforts of this group may lead to identification and validation of biomarkers that can be used in assessment of the disease-modifying capacities of established and emerging OA treatments.
CONCLUSION
Future therapies for knee OA will continue to be focused on reducing patients' pain and improving their function. However, they also will be aimed at early detection and early intervention with chondroprotection.
References:
REFERENCES
1. Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:376-378.
2. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625-635.
3. Samuels J, Krasnokutsky S, Abramson SB. Osteoarthritis: a tale of three tissues. Bull NYU Hosp Jt Dis. 2008;66:244-250.
4. Block JA, Shakoor N. The biomechanics of osteoarthritis: implications for therapy. Curr Rheumatol Rep. 2009;11:15-22.
5. Tchetina EV. Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis. 2011;2011:683970. Epub 2010 Dec 29.
6. Attur M, Samuels J, Krasnokutsky S, Abramson SB. Targeting the synovial tissue for treating osteoarthritis (OA): where is the evidence? Best Pract Res Clin Rheumatol. 2010;24:71-79.
7. Martel-Pelletier J, Pelletier JP. Is osteoarthritis a disease involving only cartilage or other articular tissues? Eklem Hastalik Cerrahisi. 2010;21:2-14.
8. Walsh DA, McWilliams DF, Turley MJ, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford). 2010;49:1852-1861.
9. Martel-Pelletier J, Kwan Tat S, Pelletier JP. Effects of chondroitin sulfate in the pathophysiology of the osteoarthritic joint: a narrative review. Osteoarthritis Cartilage. 2010;18(suppl 1):S7-S11.
10. Musumeci G, Loreto C, Carnazza ML, Martinez G. Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19:307-313.
11. Suri S, Gill SE, Massena de Camin S, et al. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66:1423-1428.
12. Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707-1715.
13. Neu CP, Reddi AH, Komvopoulos K, et al. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum. 2010;62:2680-2687.
14. Silver FH, Bradica G, Tria A. Viscoelastic behavior of osteoarthritic cartilage. Connect Tissue Res. 2001;42:223-233.
15. Moskowitz RW, Kelly MA, Lewallen DG. Understanding osteoarthritis of the knee-causes and effects. Am J Orthop (Belle Mead NJ). 2004;33(2 suppl):S5-S9.
16. Elsaid KA, Jay GD, Warman ML, et al. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1746-1755.
17. White DK, Zhang Y, Niu J, et al. Do worsening knee radiographs mean greater chances of severe functional limitation? Arthritis Care Res (Hoboken). 2010;62:1433-1439.
18. Garnero P, Aronstein WS, Cohen SB, et al. Relationships between biochemical markers of bone and cartilage degradation with radiological progression in patients with knee osteoarthritis receiving risedronate: the Knee Osteoarthritis Structural Arthritis randomized clinical trial. Osteoarthritis Cartilage. 2008;16:660-666.
19. Bruyere O, Collette J, Kothari M, et al. Osteoarthritis, magnetic resonance imaging, and biochemical markers: a one year prospective study. Ann Rheum Dis. 2006;65:1050-1054.
20. King KB, Lindsey CT, Dunn TC, et al. A study of the relationship between molecular biomarkers of joint degeneration and the magnetic resonance-measured characteristics of cartilage in 16 symptomatic knees. Magn Reson Imaging. 2004;22:1117-1123.
21. Williams FM, Spector TD. Biomarkers in osteoarthritis. Arthritis Res Ther. 2008;10:101.
22. Cheng T, Li FF, Zhao S, et al. Soluble P selectin in synovial fluid level is correlated with the radiographic severity of knee osteoarthritis. Clin Chim Acta. 2010;411:1529-1531.
23. Gao SG, Li KH, Zeng KB, et al. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthritis Cartilage. 2010;18:82-87.
24. Honsawek S, Chayanupatkul M, Tanavalee A, et al. Relationship of plasma and synovial fluid BMP-7 with disease severity in knee osteoarthritis patients: a pilot study. Int Orthop. 2009;33:1171-1175.
25. Honsawek S, Tanavalee A, Yuktanandana P. Elevated circulating and synovial fluid endoglin are associated with primary knee osteoarthritis severity. Arch Med Res. 2009;40:590-594.
26. Jacobi M, Villa V, Magnussen RA, Neyret P. MACI-a new era? Sports Med Arthrosc Rehabil Ther Technol. 2011;3:10.
27. Arthritis Foundation. News from the Arthritis Foundation. Osteoarthritis fact sheet. 2008. http://www.arthritis.org/media/newsroom/Osteoarthritis_Fact_Sheet_from_AF-Final_12-10-09.pdf. Accessed May 24, 2012.
28. March L, Cross M, Tribe K, et al. Cost of joint replacement surgery for osteoarthritis: the patients' perspective. J Rheumatol. 2002;29:1006-1014.
29. Sadosky AB, Bushmakin AG, Cappelleri JC, Lionberger DR. Relationship between patient-reported disease severity in osteoarthritis and self-reported pain, function and work productivity. Arthritis Res Ther. 2010;12:R162.
30. Ozcakir S, Raif SL, Sivrioglu K, Kucukcakir N. Relationship between radiological severity and clinical and psychological factors in knee osteoarthritis. Clin Rheumatol. 2011;30:1521-1526.
31. Arthritis Foundation/Centers for Disease Control and Prevention. A national public health agenda for osteoarthritis 2010. http://www.cdc.gov/arthritis/docs/OAagenda.pdf. Accessed May 24, 2012.
32. Hunter DJ. Pharmacologic therapy for osteoarthritis-the era of disease modification. Nat Rev Rheumatol. 2011;7:13-22.
33. Altman RD. Early management of osteoarthritis. Am J Manag Care. 2010;(16 Suppl Management): S41-S47.
34. Le Graverand-Gastineau MP. Disease modifying osteoarthritis drugs: facing development challenges and choosing molecular targets. Curr Drug Targets. 2010;11:528-535.
35. Mobasheri A, Henrotin Y. Biomarkers of osteoarthritis: opportunities and challenges for clinicians, basic scientists and the pharmaceutical industry. SciTopics. 2010. http://www.scitopics.com/Biomarkers_of_Osteoarthritis_Opportunities_and_Challenges_for_Clinicians_Basic_Scientists_and_the_Pharmaceutical_Industry.html. Accessed May 24, 2012.
36. Ornetti P, Brandt K, Hellio-Le Graverand MP, et al. OARSI-OMERACT definition of relevant radiological progression in hip/knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:856-863.
37. Emrani PS, Katz JN, Kessler CL, et al. Joint space narrowing and Kellgren-Lawrence progression in knee osteoarthritis: an analytic literature synthesis. Osteoarthritis Cartilage. 2008;16:873-882.
38. Deberg M, Dubuc JE, Labasse A, et al. One-year follow-up of Coll2-1, Coll2-1NO2 and myeloperoxydase serum levels in osteoarthritis patients after hip or knee replacement. Ann Rheum Dis. 2008;67:168-174.
39. Osteoarthritis Research Society International. OARSI initiatives. OABiomarker Global Initiative. http://www.oarsi.org/index2.cfm?section=OARSI_Initiatives&content=Biomarkers. Accessed May 24, 2012.




