Article
Managing meniscal injuries: The treatment
Better understanding of meniscus anatomy and function has triggered a search for new and improved treatment options. Nonoperative treatment, including activity modification, rehabilitation, and use of NSAIDs, is directed at minimizing symptoms of pain and swelling.
Absence of the meniscus, once thought to be a vestigial organ, is now known to lead to a recognizable pattern of joint deterioration, including joint-space narrowing, osteophyte formation, and squaring of the femoral condyles.1 Appreciation of meniscal function has led to use of surgical interventions beyond partial and total meniscectomy. Increased understanding of meniscus anatomy and function has driven medical practitioners to search for more flexibility in treatment of the injured meniscus.
Arthroscopic treatment of meniscal injuries is one of the most common orthopedic procedures performed in the United States.2 With the use of arthroscopy, surgeons are attempting more aggressive procedures to salvage or replace the injured meniscus. Treatment has evolved dramatically with the use of arthroscopy and will continue to change as alternative options are developed and perfected.
In this 2-part article, we describe the diagnosis and management of meniscal injuries. In the first part (The Journal of Musculoskeletal Medicine, September 2009, page 343), we reviewed the anatomy and function of the meniscus, the epidemiology, common tear patterns, and the elements of the physical examination and imaging that lead to the diagnosis. In this second part, we describe nonoperative and operative approaches to treatment, including recent advances in meniscal regeneration and transplant.
Nonoperative treatment
When selecting an appropriate treatment for patients with meniscal injury, physicians should consider both patient factors and tear characteristics. Factors that help decide whether to use conservative or operative treatment include chronicity of symptoms, tolerance for activity modification, tolerance for risk of failure, expectations, age, and condition of the joint.3
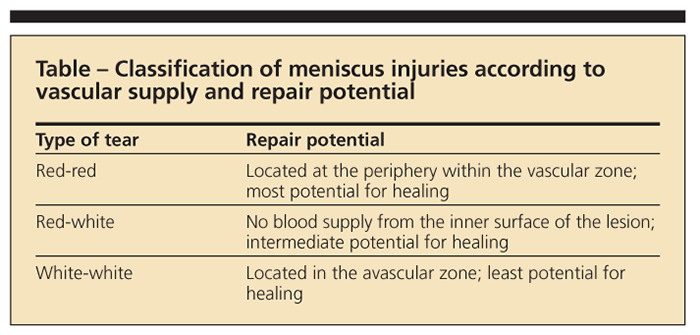
The configuration, and especially the location, of the tear is a major determinant of successful healing. Only the peripheral one-third of the meniscus has a vascular supply and, therefore, a higher potential capacity to heal.3,4 The middle 70% to 80% of the central meniscus has inferior conditions for healing because of the lack of blood supply. The tear pattern and location must be appropriate to be considered for repair. In addition to grading of meniscal signals as shown by MRI, meniscus tears may be classified according to their vascular supply to predict the repair potential (Table).3,5 When the tear is too complex, degenerative, or in an avascular portion of the meniscus, surgical treatment will require partial meniscectomy.
Although surgical treatment is recommended for most meniscal tears, those that cause minor symptoms in low-demand patients may be managed conservatively. Nonoperative treatment is directed at minimizing symptoms of pain and swelling; it should consist of a trial of activity modification, rehabilitation, and the use of therapeutic doses of NSAIDs. Patients should be advised that symptoms reoccur frequently.
Rehabilitation should focus on maintenance of full range of motion and progressive conditioning as allowed without exacerbation of symptoms. Various modalities are available to therapists to help control effusions, including ultrasonography, electric stimulation, and cold therapy.
Arthroscopy
Indications for surgical intervention include symptoms that affect activities of daily living, work, or sport; inadequate response to nonsurgical management; and absence of other causes of knee pain identified by radiography or other imaging. Arthroscopy is the mainstay of surgical treatment (Figure).
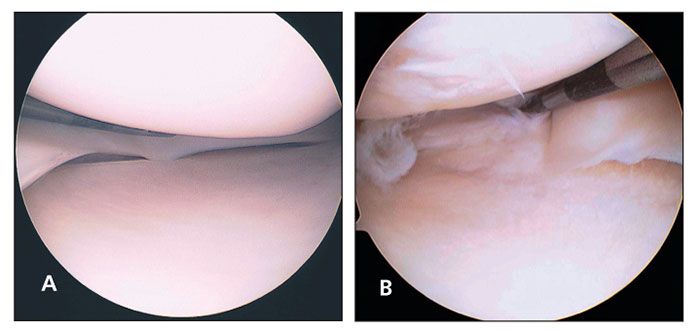
Figure –The normal arthroscopic appearance of the meniscus is shown here (A). This arthroscopic visualization (B) shows a complex medial meniscus tear with radial and horizontal patterns.
The goal of meniscal surgery is to provide a mechanically stable meniscus while maximizing meniscal preservation. This is accomplished by repairing amenable tears that have an adequate blood supply to allow for healing and by removing the irreparable portions of the meniscus that are unstable.
Not all tears found with arthroscopy require intervention. Stable tears have the potential capacity to heal without treatment; they include those shorter than 10 mm, those that have less than
3-mm displacement on arthroscopic probing, partial-thickness tears (less than 50% of meniscal depth), and radial tears shorter than 3 mm.3 Abrasion of the tear and synovium with arthroscopic rasping or trephination of tears to encourage neovascularization may promote healing; these techniques may be successful in up to 90% of cases.3,6
Results of early treatment of peripheral tears (suspected with development of an effusion during the first 48 hours) are improved with surgical repair performed within the first 4 months from the time of injury (ideally, less than 10 weeks).6 Additional predictors of favorable outcome from repair include location (tear located within 3 mm of the meniscosynovial junction), patient age younger than 30 years, tear length less than 2.5 cm, tear of the lateral rather than medial meniscus, and simultaneous anterior cruciate ligament (ACL) reconstruction (resulting from intra-articular bleeding and fibrin clot formation from the reconstruction portion of the procedure).5-7
Displaced tears that result in a block to motion should be managed expeditiously. These tears, termed "bucket handle tears," typically involve the majority of the meniscus.
Precocious arthropathy may result from partial or total meniscectomy because of the increased contact stresses on the articular surfaces. Total meniscectomy in previously normal knees results in significant arthrosis in two-thirds of patients within 15 years after the surgery. 8 Improved outcome is noted in knees after successful repair; the incidence of degenerative changes is decreased after 5 years.3
More rapid degeneration is noted after lateral meniscectomy than after medial meniscectomy. 3,9,10 Articular cartilage damage present at the time of partial meniscectomy is the factor that has the biggest impact on long-term outcome.6 Functional results do not always correlate with radiographic findings. Up to 50% of patients demonstrate radiographic degeneration at 8 years after partial meniscectomy, compared with 25% of patients who did not receive treatment.2,3, 9
Partial meniscectomy
This is the most common surgical procedure for managing meniscal tears, because the blood supply required for healing is limited and most often there is underlying degeneration of the substance of the meniscus, rendering it irreparable. Meniscal tears that do not fall into the category of stable (having potential for spontaneous healing) or repairable are best managed with partial meniscectomy to remove unstable fragments, eliminate mechanical symptoms, avoid propagation of the tear, and reduce pain and swelling.
The goals of partial meniscectomy are to remove nonfunctioning tissue, maximize meniscus preservation, and create a stable configuration of the remaining tissue. Indications for this type of treatment include complete oblique, radial, or horizontal tears; degenerative or complex tears; and tears that are located in the white-white zone (central zone with no blood supply on either side of the tear).3
Outcomes of partial meniscectomy remain good or excellent in more than 90% of patients who do not demonstrate articular cartilage damage at the time of meniscectomy; if damage is present, these outcomes are seen in only 60% of patients.6 Other factors that influence risk of future arthritis include the amount of resection (greater resection results in higher risk), type of resection (radial resection eliminates the ability of the meniscus to convert compressive forces to hoop stresses and renders the meniscus incompetent), associated knee instability, overall weight-bearing alignment, body habitus, age, and activity level. Overall, 80% to 90% of patients have good to excellent results within the first 5 years after partial meniscectomy.3
The benefit of partial meniscectomy in patients with radiographic evidence of degenerative disease is debated. The outcomes for this surgery are variable. Patients with grade IV osteoarthritis had good results from partial medial meniscectomy at 2-year follow-up when the symptoms that prompted the surgery were mechanical, there was a preserved joint space on
45° flexion weight-bearing radiographs, and there was a normal standing alignment of the limb.11
Meniscal repair
Approaches to repair include open, arthroscopically assisted, and all-arthroscopic repair. Traditional techniques employ suture fixation using a variety of devices that are tied through a posterior counterincision. Meniscus repair implants have continued to evolve since their introduction in the mid-1990s. Implants in the current generation incorporate a suture-based repair and allow for an all-arthroscopic technique.
Criteria for meniscus repair include a complete vertical-longitudinal tear that is longer than 10 mm, location within the peripheral 10% to 30% of the meniscus (or within 3 to 4 mm of the meniscosynovial junction), displacement of more than 3 to 5 mm on arthroscopic probing, no significant joint degeneration or deformity, and a stable knee.3,5,6 It may be appropriate to extend the indications in younger patients and in patients in whom resection would lead to nonfunctional remaining tissue.3
When appropriate criteria are used in making the decision for repair, it is successful in about 80% of cases. In the setting of concomitant ACL reconstruction, healing rates increase to about 95%,6,12 allowing most athletes to return to full activity after meniscal repair.13 Better outcomes are noted with successful repair than with resection; the incidence of degenerative change after 5 years is lower.3
Meniscal repair has more potential complications than partial meniscectomy-specifically, neurovascular injuries. The peroneal nerve is at greatest risk during lateral meniscus repair; the popliteal artery, popliteal vein, and tibial nerve also are at risk. Medial repair includes the risk of injury to the saphenous nerve, particularly the infrapatellar branch. Failure of repair resulting in the need for additional procedures is another risk.
Meniscal substitutes
Substitutes for injured or lost meniscus tissue are gaining clinical popularity. The goal of meniscal restoration is to protect the articular cartilage and delay the onset of arthritic changes. Current options include meniscal allograft transplant and collagen meniscal implants. Meniscal allograft transplant has been in use in humans for more than 15 years. The allograft tissue most commonly used is fresh-frozen, or cryopreserved.6 Immune response against the transplant has been shown, but frank rejection rarely occurs. The technique has proved to be reproducible in terms of healing and control of postmeniscectomy pain and swelling. However, the exact indications continue to evolve.10
Transplant may be considered for patients who have symptoms referable to a meniscus-deficient tibiofemoral compartment, including pain and swelling, rather than mechanical symptoms.3,10 Transplant of the lateral meniscus is considered when there is less tissue loss than the medial meniscus because of its greater role in load transmission.14 Transplant results are poor in cases of advanced joint degeneration and, therefore, transplant should be considered only when no more than fibrillation and fissuring of the articular surface are present. Full-thickness articular cartilage lesions on the flexion weight-bearing zone of the femoral condyle or tibia greater than 10 to 15 mm in diameter are a contraindication to transplant.6,10
An additional requirement for allograft surgery is a stable knee without malalignment. An unstable or malaligned knee must be corrected to avoid direct weight bearing through the involved compartment receiving the meniscus transplant.3,10 When properly indicated, transplant leads to good results in most patients.3
Meniscal transplants can be performed in combination with other procedures around the knee to better restore the normal alignment and mechanics to allow for increased survival of the meniscal transplant. When malalignment is present, meniscal transplant can be performed in conjunction with an osteotomy. This has been shown to lead to improved functional outcome scores.14
Medial meniscal transplant is indicated at the time of ACL reconstruction when the medial meniscus is deficient. The medial meniscus acts as a secondary restraint to anterior translation; restoring its function with transplant has led to improved anterior stability as well as graft survival.14
Meniscal transplant may be considered in patients with chondral damage if it is performed in combination with chondral resurfacing. Osteochondral allograft or autograft may be used, depending on the nature and size of the cartilaginous defect.
Microfracture (creating small holes in the subchondral bone with an arthroscopic awl to encourage fibrocartilage ingrowth) is used for defects smaller than 10 mm with normal surrounding cartilage. These procedures performed in concert serve a dual purpose: restoration of the articular surface makes the patient a candidate for meniscal transplant, and the restored meniscal function protects the chondral surface during healing and, theoretically, leads to improved survival.14 The rate of patient satisfaction after meniscal transplant performed in conjunction with articular cartilage repair has been reported at 76%.15
Patients who undergo significant partial meniscectomy (subtotal meniscectomy) but have a retained intact rim of meniscal tissue may be candidates for a collagen meniscal implant (CMI), also known as a bovine collagen meniscal scaffold. CMI has been approved for human implantation in Europe, Australia, and Chile; now it is available in the United States.
CMI is a collagen-based implant made from bovine Achilles tendon–derived collagen and other sources. The collagen has undergone physical and chemical modifications, as well as shaping, to form a scaffold that acts as a temporary replacement for lost meniscal tissue. The scaffold is gradually replaced by meniscus-like tissue as fibrochondrocytes proliferate within the scaffold.16 At second-look arthroscopy after CMI, 69% of the original defect appeared to be filled with fibrocartilage tissue.16
In preliminary results, collagen meniscal scaffolds have shown promise as an alternative to allograft transplant. Patients note subjective improvement in symptoms, and generation of meniscus-like tissue is seen on arthroscopy and histological examination.17
One advantage of CMI is its availability. The CMI scaffolds are intraoperatively sized and contoured and do not require the exact size matching that accompanies the use of allograft tissue. Allograft transplant requires that the donor graft is within 5% of the exact size of the native tissue, creating a need for precise preoperative measurements and, often, a significant wait period until a size-matched donor is available.
Polyurethanes currently are being investigated as alternatives to CMI. They are thought to have better mechanical properties for suturing to the remaining meniscus, and they are more resistant to the forces the implant is subject to in the knee joint. Prospective trials are under way to investigate their use. Animal studies have shown decreased joint contact forces after replacement with polyurethanes compared with partial meniscectomy alone.16
Future treatments
Meniscus repair and replacement treatments continue to evolve. Animal studies have shown that intra-articular injected synovial stem cells have the ability to differentiate into meniscal cells and promote regeneration of meniscal defects.18 Several growth factors and cytokines are under investigation as possible adjuncts to promote healing. Meniscal fibrochondrocytes respond with migration and proliferation to growth factors, including platelet-derived growth factor, hepatocyte growth factor, bone morphogenic protein–2, insulinlike growth factor–1, and transforming growth factor-β.19,20 Suggested delivery systems for growth factors include impregnated absorbable scaffolds, impregnated fixation devices, and even virus vectors for gene therapy.21 Tissue engineering may be the next step in development of a durable meniscal replacement.
References:
References1. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg. 1948;30B:664-670.
2. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury, I: basic science and evaluation. J Am Acad Orthop Surg. 2002;10:168-176.
3. Klimkiewicz JJ, Shaffer B. Meniscal surgery 2002 update: indications and techniques for resection, repair, regeneration, and replacement. Arthroscopy. 2002;18(9 suppl 2):14-25.
4. Arnoczyk S, McDevitt C. The meniscus: structure, function, repair, and replacement. In: Buckwalter JA, Einhorn TA, Simon SR, eds. Orthopaedic Basic Science: Biology and Biomechanics of the Musculoskeletal System. 2nd ed. Rosemont, IL: AAOS; 2000:531-545.
5. DeHaven KE. Meniscus repair. Am J Sports Med. 1999;27:242-250.
6. Greis PE, Holmstrom MC, Bardana DD, Burks RT. Meniscal injury, II: Management. J Am Acad Orthop Surg. 2002;10:177-187.
7. Eggli S, Wegmüller H, Kosina J, et al. Long-term results of arthroscopic meniscal repair: an analysis of isolated tears. Am J Sports Med. 1995;23:715-720.
8. Andersson-Molina H, Karlsson H, Rockborn P. Arthroscopic partial and total meniscectomy: a long-term follow-up study with matched controls. Arthroscopy. 2002;18:183-189.
9. Jaureguito JW, Elliot JS, Lietner T, et al. The effects of arthroscopic partial lateral meniscectomy in an otherwise normal knee: a retrospective review of functional, clinical, and radiographic results. Arthroscopy. 1995;11:29-36.
10. Rodeo SA. Meniscal allografts-where do we stand? Am J Sports Med. 2001;29:246-261.
11. Bin SI, Lee SH, Kim CW, et al. Results of arthroscopic medial meniscectomy in patients with grade IV osteoarthritis of the medial compartment. Arthroscopy. 2008;24:264-268.
12. Toman CV, Dunn WR, Spindler KP, et al. Success of meniscal repair at anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37:1111-1115.
13. Logan M, Watts M, Owen J, Myers P. Meniscal repair in the elite athlete: results of 45 repairs with a minimum 5-year follow-up. Am J Sports Med. 2009;37:1131-1134.
14. Packer JD, Rodeo SA. Meniscal allograft transplantation. Clin Sports Med. 2009;28:259-283, viii.
15. Rue JP, Yanke AB, Busam ML, et al. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36:1770-1778.
16. van Tienen TG, Hannink G, Buma P. Meniscus replacement using synthetic materials. Clin Sports Med. 2009;28:143-156.
17. Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of meniscal cartilage with use of a collagen scaffold: analysis of preliminary data. J Bone Joint Surg. 1997;79A:1770-1777.
18. Horie M, Sekiya I, Muneta T, et al. Intra-articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27:878-887.
19. Bhargava MM, Attia ET, Murrell GA, et al. The effect of cytokines on the proliferation and migration of bovine meniscal cells. Am J Sports Med. 1999;27:636-643.
20. Ochi M, Uchio Y, Okuda K, et al. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy. 2001;17:724-731.
21. Martinek V, Usas A, Pelinkovic D, et al. Genetic engineering of meniscal allografts. Tissue Eng. 2002;8:107-117.




