Article
Multibiomarker Disease Activity Scores Support Assessment of Biosimilarity in RA
Author(s):
This MBDA score has been validated to correlate with the disease activity score, including a 28-joint count and CRP (DAS28-CRP), in patients with rheumatoid arthritis.
Data from 2 randomized controlled trials (RCTs) explored the use of the multibiomarker disease activity (MBDA) score to supplement biosimilarity assessments in patients with rheumatoid arthritis (RA). Based on the results, MBDA scores have the potential to provide objective and quantitative evidence of biosimilarity, without the subjectivity associated with patient or physician assessments and joint counts, according to a study published in Rheumatic & Musculoskeletal Diseases.
“The MBDA score (Vectra, Myriad Genetics, Salt Lake City, Utah, USA) has been validated to provide a quantitative measure of disease activity in patients with RA,” investigators stated. “Using a proprietary algorithm, the concentrations of 12 biomarkers, including C reactive protein (CRP), are combined to calculate a score that ranges from 1 to 100. This MBDA score has been validated to correlate with the disease activity score that includes a 28-joint count and CRP (DAS28-CRP) in patients with RA.”
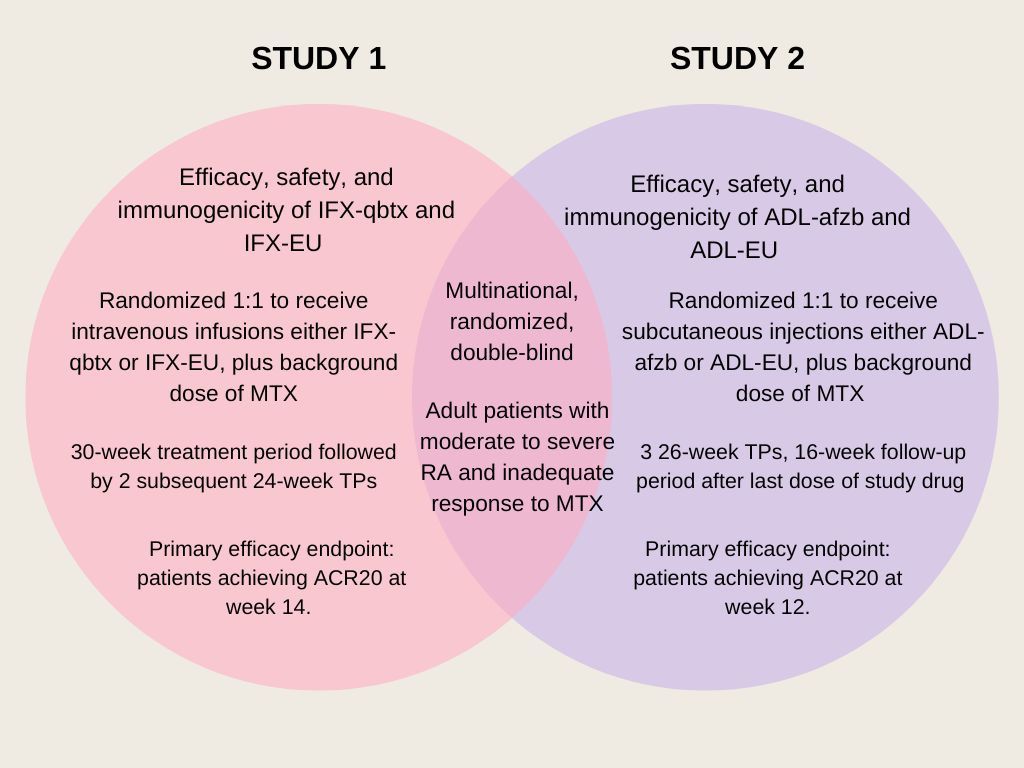
Of the 2 RCTs analyzed, both of which were multinational, randomized, and double-blind studies, 1 evaluated the efficacy, safety, and immunogenicity of the biosimilar infliximab (IFX-qbtx) and the other examined the same endpoints for the biosimilar adalimumab (ADL-afzb). Patients enrolled in both studies were adult patients with active RA who met the 2010 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology classification criteria for RA for ≥4 months as well as the 1991 revised ACR criteria for functional status classes I–III.
In the first study, 560 patients were randomized 1:1 to receive IFX-gbtx or European Union-sourced infliximab (IFX-EU) 3 mg/kg intravenous at weeks 0, 2, 6, and then every 8 weeks. The second study randomized 597 patients 1:1 to receive either ADL-afzb or adalimumab (ADL-EU) 40 mg subcutaneous every other week. All treatments were taken with a stable background dose of methotrexate (MTX) of 10-25 mg/week. Mean MBDA scores were calculated at baseline as well as timepoints throughout the treatment period 1 (TP1) of both IFX (weeks 6, 14, 30) and ADL (weeks 6, 12, 26).
Mean baseline MBDA scores were 61.3 (±12.5) and 58.8 (±13.2) for patients in the IFX-qbtx cohort (n = 236) and IFX-EU cohort (n = 248), respectively. Baseline scores were 57.2 (±14.44) for the ADL-afzb group (n = 292) and 58.3 (±15.34) for the ADL-EU group (n = 293). All mean MBDA scores, changes from baseline in MBDA scores, and American College of Rheumatology (ACR) 20% improvement (ACR20) response rates were similar between the biosimilar and reference product at all timepoints in both studies. Although mean MBDA scores plateaued at week 6, ACR response rates and DAS28-CRP improved throughout the study periods.
The limited number of timepoints included in the assessments, such as not evaluating MBDA scores before week 6, is a limitation attributed to the exploratory analysis nature of the study. Earlier timepoints may have been more sensitive and helpful in identifying possible differences between biosimilars and reference products. Further, primary endpoints based on the proportion of patients achieving ACR20 responses were used to determine the power calculations as opposed to the MBDA scores. However, similar trends in mean MBDA scores were seen in both studies.
“The MBDA score may provide objective, quantitative and potentially earlier evidence of biosimilarity using an assessment of disease activity that is independent of the potential subjectivity inherent to counts of tender joints or global assessments of disease activity by the patient or assessor,” investigators concluded.
Reference:
Kay J, Bock AE, Rehman M, et al. Use of multibiomarker disease activity scores in biosimilarity studies for the treatment of patients with rheumatoid arthritis. RMD Open. 2022;8(2):e002423. doi:10.1136/rmdopen-2022-002423




