Article
New Guidance Stipulates When to Use UV Phototherapy for Skin Disorders
Author(s):
The authors unanimously agreed that doctors should explain the potential benefits and negatives of the treatment and all centers should have a phototherapy protocol in place to address episodes of symptomatic erythema and other adverse events.
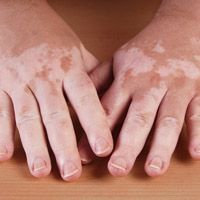
As narrowband ultraviolet B phototherapy (NB-UVB) becomes more prevalent, investigators have put plans in place on when the treatment should be used for skin diseases and what facilities and clinicians can do to improve care.
A team, led by Victoria Goulden, MD, Leeds Teaching Hospitals NHS Trust, developed new evidence-based guidance for using narrowband ultraviolet B phototherapy for patients of all ages.
“UV radiation has been shown to have potent immunomodulatory properties, which involve multiple mechanisms in both the innate and adaptive immune systems,” the authors wrote. “It has a significant immunosuppressive effect on T-cell function and induces antigen-specific tolerance that depends on interactions between antigen-presenting cells, mast cells and keratinocytes.”
Making the Reccomendations
The investigators developed the new guidelines using the BAD’s recommended methodology and established several clinical questions related to the scope of the guideline, as well as a set of outcome measures of importance to patients, ranked using the GRADE methodology.
The Guideline Development Group included 10 consultant dermatologists, a medical physicist, a phototherapy nursing sister, 3 patient representatives and a technical team consisting of an information scientist, a guideline research fellow, and a project manager providing methodological and technical support.
The team conducted a systematic literature search to identify key articles on NB-UVB up to February 18, 2021.
Key Questions
The first review questions focused on individuals with skin diseases, including psoriasis, vitiligo, eczema, hand and foot dermatoses, lichen planus, mycosis fungoides, pityriasis lichenoides, subacute and nodular prurigo, pruritus, chronic spontaneous urticaria, alopecia areata, progressive macular hypomelanosis and morphea/localized scleroderma.
The investigators wanted to know the clinical efficacy, safety, and tolerability of NB-UVB phototherapy, as either a monotherapy or in combination with another treatment, compared to other light-based therapies such as the excimer laser and lamp, topical therapy, retinoid therapy, conventional systemic immunosuppression or immunomodulation, biological therapy, placebo, no treatment or NB-UVB in combination with a different treatment.
The second clinical question focused on patients with photodermatoses, similarly looking at the efficacy, safety, and tolerability of the treatment.
General Guidelines
The investigators unanimously agreed that doctors should explain the potential benefits and negatives of the treatment with an information leaflet and all centers should have a phototherapy protocol in place to address episodes of symptomatic erythema and other adverse events.
The recommendations also call for minimal erythema dose testing or testing a small area prior to starting treatment to identify a safe starting dose for NB-UVB, evaluating all phototherapy devices and checking safety by medical physicals and carrying out irradiance measurements at regular intervals appropriate for the frequency of use.
Centers should also consider providing a home phototherapy service and offer skin cancer surveillance at appropriate intervals to individuals identified as having received more than 500 whole-body NB-UVB treatments. This is especially true for individuals with other coexisting risk factors for skin cancer.
On the other hand, the authors suggest not offering phototherapy to individuals taking various drugs, including ciclosporin, mycophenolate, azathioprine, or oral tacrolimus.
The final 2 general recommendations included daily use of an emollient during the course of treatment to prevent and alleviate skin dryness and pruritus but avoiding emollient use for at least 2 hours prior to NB-UVB use, especially for patients with psoriasis.
The authors also provided specific guidance for treating psoriasis, vitiligo, eczema, palmoplantar dermatoses, lichen planus, morphea, mycosis fungoides, pityriasis lichenoides, progressive macular hypomelanosis, subacute and nodular prurigo, photodermatoses, pruritus, and chronic urticaria.
The guidance for specific diseases often focuses on timing of treatment, as well as what to do when the individual is being treated with other treatments for their condition.
The investigators also made a number of recommendations for future randomized controlled trials, including for hyperkeratotic plaque psoriasis, lichenified eczema, nodular prurigo, palmoplantar psoriasis, palmoplantar eczema, and generalized granuloma annulare.
The study, “British Association of Dermatologists and British Photodermatology Group guidelines for narrowband ultraviolet B phototherapy 2022,” was published online in the British Journal of Dermatology.





