Article
New PDE4 Inhibitor Conquers PsA in Phase 3 Trial
ACR2013: Apremilast, a new kind of anti-inflammatory, proved significantly better than placebo against psoriatic arthritis after 52 weeks. Adverse effects were short-lived. Results for ustekinumab also held up after a year.
Apremilast, a new small-molecule tyrosine kinase inhibitor that blocks the enzyme phosphodiesterase-4 (PDE-4) important in inflammation, has proven effective against psoriatic arthritis after 52 weeks, in results from Phase 3 trials reported to an over-capacity audience at the American College of Rheumatology annual meeting in San Diego.
The most noteworthy side effects are nausea and vomiting, which affect less than 5% of patients in clinical trials and usually subside within a week.
One of the new class of biologics that affect signaling within rather than between immune cells, apremilast has been tested in a three-trial series of multi-national studies known by the acronym PALACE.
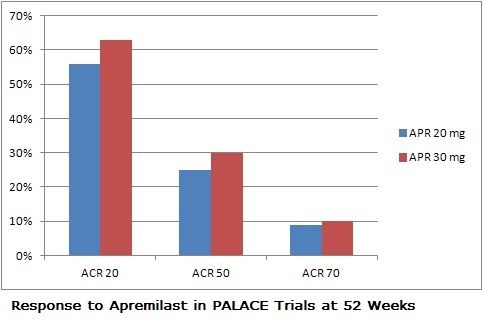
The trials randomized 505 patients with well-documented psoriatic arthritis (and with skin manifestations in PALACE-3) to receive either placebo, 20 mg of apremilast BID, or 30 mg BID. At 16 weeks placebo subjects who had not improved were randomized to either dose of the drug, and all placebo patients were randomized to the drug at week 24. Their results were comparable to patients given the drug from the outset.
Patients were allowed to continue with concurrent disease-modifying antirheumatic drugs (DMARDs) during the trial.
In all 3 PALACE trials, apremilast achieved statistically meaningful improvements in ACR20 response (the primary endpoint) compared to placebo, results which were maintained over 52 weeks. The drug was also associated with improvements in the severity of enthesitis and dactylitis. The median change in the MASES (Maastricht Ankylosing Spondylitis Enthesitis Score) index was 66.7% with either dose of apremilast. About 40% of patients on the drug saw complete resolution of enthesis during the trial, and dactylitis disappeared in about two-thirds of patients within the year of treatment.
Adverse events included diarrhea, nausea, headache, and vomiting, which affected less than 5% of all patients who took the drug.Less than 3% of patients discontinued the drug during the trials due to these effects, which reduced after the first week of dosing.
A chemically different PDE4 inhibitor, roflumilast (Daliresp), is FD- approved for the treatment of chronic obstructive pulmonary disease. According to Kamal Shah, head of pharmacovigilance at Cellgene (which developed apremilast), seven other PDE4 inhibitors have been tested for rheumatic conditions, but all have been abandoned, largely due to concerns about adverse events.
Shah said the company expects approval for the psoriatic arthritis indication in March 2014.
In a Phase 2 trial of apremilast also reported at the ACR meeting, the PDE4 inhibitor proved rapidly and significantly powerful against oral and genital ulcers among adults with Behet's syndrome. Oral ulcers disappeared completely for 71% of patients who took the drug for 12 weeks (but for only 29% of those randomized to placebo), and the rare cases of genital ulcers also resolved completely on the drug. Adverse events were similar to those in the PsA trials.
Also reported at the ACR meeting were 52-week results for the multicenter randomized PSUMMIT-2 trial of ustekinumab, which has been approved for treatment of PsA. Also a crossover trial, PSUMMIT-2 allowed subjects with less than 5% improvement in swollen joint counts to take 45 mg of ustekinumab at 16 weeks.
Ustekinumab maintained similar efficacy at 52 weeks against psoriatic arthritis as it had at 24 weeks, although the response was more robust in patients who had never taken a TNF inhibitor, and less so but still significant for people who had previously taken fewer than three TNF inhibitors.
The drug was generally well tolerated over 60 weeks, the researchers report, although two patients (both of whom had previously taken TNF inhibitors) developed malignancies and two others had serious infections while taking the drug.




