Article
New Therapeutic Options for Gout Here and On the Horizon
ABSTRACT: No new drug was FDA-approved for gout for close to 45 years, but new drugs are on the market now and others are in development. Established treatments often are effective, but each has limitations. In 2009, the FDA approved a nongeneric colchicine for acute gout.
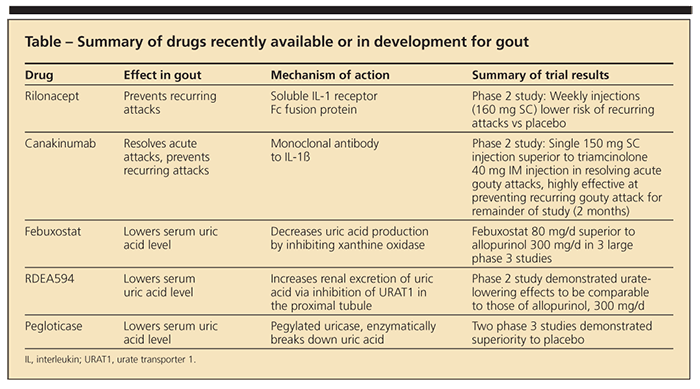
Gout is the most common inflammatory arthritis in the United States (Figure), with a prevalence of about 3%,1 and the number of persons with gout has increased over the past few decades. Until recently, however, gout management had not kept up with the rising tide. Between 1965 and early 2009, not a single new drug was approved for gout. Now, treatment is poised for a transformation. New drugs are on the market, and others are in clinical development (Table). In this article, we review newly FDA-approved treatments for patients with gout and the potential utility of several agents in the pipeline.
Figure – The prevalence of gout has increased over the past few decades. Gouty arthropathy usually presents as an acute monarticular arthritis, with initial attacks occurring primarily in the feet in men. Symptoms include joint pain, swelling, redness, and warmth.
NEW AGENTS FOR MANAGING OR PREVENTING GOUTY INFLAMMATION
Established treatments for patients who have acute gout-high-dose NSAIDs, high-dose oral colchicine, and oral or injectable (intra-articular, depot intramuscular) corticosteroids-are valid and often effective approaches to managing acute gouty arthropathy. However, each has limitations, including adverse effects that can potentiate, or be potentiated by, comorbidities in a patient with gout.
For example, any NSAID (including but not limited to indomethacin) may abrogate a gout attack, but many patients with gout carry 1 or multiple relative contraindications to this class of drugs, such as hypertension and renal insufficiency (Pillinger MH, Keenan R, unpublished data). Similarly, the use of oral corticosteroids is relatively contraindicated in patients with diabetes mellitus, and high-dose colchicine or NSAIDs should not be prescribed for patients with moderate to severe renal insufficiency. Thus, there is a need for alternative therapies that have novel mechanisms or different safety profiles or both.
Colchicine
This drug, known to Hippocrates, has been in modern use for gout since 1810. However, colchicine had never received FDA approval as gout therapy until recently.
With this deficiency in mind, the FDA countenanced a reexamination of colchicine in acute gout. This led to FDA approval of a nongeneric colchicine (Colcrys) in 2009, after a clinical trial demonstrated that 1.2 mg of oral colchicine, followed by 0.6 mg 1 hour later, is at least as successful in resolving acute gouty attacks as the previously used high-dose hourly regimen, with a dramatically improved adverse-effect and safety profile.2
The new regimen may be used in patients with renal or hepatic failure, although in these situations treatment should not be repeated for about 2 weeks. Although obviously useful, both this new treatment regimen and the older one are successful in just under half of patients by 24 hours, leaving more than 50% of patients in need of other treatment. Of note, the Colcrys study did not include persons with severe, polyarticular, well-established gouty attacks, which clinicians have long recognized to respond poorly to even the more traditional, higher-dose therapy.
Anti–interleukin (IL)-1β therapy
Recent studies have emphasized the centrality of IL-1β in the pathogenesis of gout. Monosodium urate crystals may activate the inflammasome, an intracellular enzyme complex that converts inactive pro–IL-1β into active IL-1β for secretion.3 With this in mind, investigators have begun to explore anti–IL-1β biologic agents for the management or prevention of gouty inflammation.
Anakinra, an IL-1β receptor antagonist that is FDA-approved for rheumatoid arthritis, has been studied off-label in patients with acute gout. In an open-label trial, anakinra improved patient-reported symptoms by at least 50% (within 48 hours) in all 10 patients enrolled.4 Patients in the trial had been unsuccessful with or intolerant of other pharmacotherapies.
Rilonacept, a soluble IL-1 receptor-Fc fusion protein, is being investigated for both treatment and prophylaxis of gouty inflammation. Terkeltaub and associates5 reported the results of a 14-week, single-blind, nonrandomized, placebo run-in trial of 10 patients with severe gout and chronic persistent gouty arthritis. Patients received placebo for the first 2 weeks and subcutaneous rilonacept once weekly in weeks 3 through 8 and then were withdrawn from treatment. Observations were made at baseline, at the end of the placebo run-in period (internal control), every 2 weeks during active treatment, and again at 14 weeks.
Several end-point improvements had been reached at the end of active treatment, including improvements in visual analogue scale pain, patient global, and high-sensitivity C-reactive protein test scores. Fifty percent of enrollees who received rilonacept therapy self-reported at least a 75% improvement in pain.5 A separate study that was presented at the 2009 annual meeting of the American College of Rheumatology (ACR) showed rilonacept to be effective for gout prophylaxis.
Schumacher and colleagues6 reported results from a randomized, double-blind, placebo-controlled trial that investigated the efficacy of weekly subcutaneous rilonacept for preventing gouty attacks during initiation of urate-lowering therapy with allopurinol. At the end of the study, only 22% of patients taking rilonacept had experienced attacks, compared with 74% of placebo recipients.6
Canakinumab, a fully human monoclonal anti–IL-1β antibody, also is under study as a treatment for patients with acute gout attacks and prevention of recurring attacks. Results from a dose-ranging, double-blinded, active-controlled trial were presented at the 2009 ACR meeting. Canakinumab (150 mg injected subcutaneously once) was superior to triamcinolone acetate (40 mg injected intramuscularly) for abrogating acute gout attacks. The median time to 50% improvement was 24 hours for canakinumab versus 48 hours for triamcinolone. In addition, patients who received canakinumab (T1/2 = 28 days) were 94% less likely to experience a recurrent attack over the following 2 months than those treated with triamcinolone (3.7% probability with canakinumab vs 45.4% with triamcinolone).7
It is unclear what role IL-1 antagonists would play in clinical practice if approved for gout. As biologic agents, they may be prohibitively more expensive than the already-available anti-inflammatory agents colchicine, NSAIDs, and corticosteroids for long-term use. On the other hand, their cost may not be excessive for management of acute attacks, particularly in patients who cannot tolerate the established medications.
NEW AGENTS FOR MANAGING HYPERURICEMIA
The root cause of gout-hyperuricemia-usually needs to be addressed to truly modify the course of disease in patients with recurring gouty arthropathy. Urate lowering also is necessary when dissolution of tophi is desired and may be advisable in patients with uric acid renal stones. Several agents in the pipeline may provide viable alternatives to the currently available urate-lowering drugs.
Urate transporter 1 (URAT1) inhibitors
Drugs to increase urate excretion have been available for decades. Probenecid and benzbromarone both inhibit the organic anion transporter URAT1, blocking urate reabsorption from the proximal renal tubule. Probenecid requires intact renal function and twice-daily dosing and is less effective than benzbromarone8; benzbromarone is unavailable in the United States because there are concerns about hepatotoxicity.
Clinical trials of a novel URAT1 inhibitor, RDEA594, are under way; at the 2009 ACR meeting, results from a small 2-week proof-of-concept study demonstrated significant hypouricemic effects. The company that is testing RDEA594 is developing another URAT1 inhibitor, RDEA684, which has 170-fold greater potency against URAT1.9
One issue of potential concern with all uricosuric agents is the possibility that increased uric acid delivery to the renal pelvis will increase the risk of kidney stones. For this and other reasons, uricosuric agents probably should not be used in patients who have a history of uric acid stones or who overexcrete uric acid at baseline. Of note, however, some stone experts are of the opinion that renal pH, rather than uric acid concentration, is the major determinant of stone formation and that patients at risk for stones may receive uricosurics as long as their urine is adequately alkalinized.10 Further research will be required to establish whether the current practice of avoiding uricosurics in stone-formers is unnecessarily conservative.
Febuxostat
A second approach to treatment, reducing the production of uric acid, may be achieved with allopurinol, a purine-based xanthine oxidase inhibitor first marketed for gout in 1963. Allopurinol typically is dosed once daily, although higher doses typically are given twice daily. In general, allopurinol is started at a low dose and titrated up, based on serum uric acid level. The agent is effective for both urate overproducers and urate underexcreters, and it has been the unequivocal drug of choice for uric acid overproducers, uric acid stone-formers, and patients with tophaceous gout.
Although allopurinol can be a very effective drug, it has limitations. Some patients are intolerant of allopurinol, which may cause a hypersensitivity syndrome that in some cases may be fatal. Patients with renal insufficiency may be at increased risk for allopurinol hypersensitivity syndrome. In addition, although allopurinol is approved for dosages up to 800 mg/d, by tradition most physicians prescribe no more than 300 mg/d, a dosage at which many patients do not achieve a desirable serum urate level (lower than 6 mg/dL).11
Febuxostat, a nonpurine xanthine oxidase inhibitor, gained FDA approval in 2009 (at dosages of 40 and 80 mg/d) for the management of hyperuricemia in patients with established gout. Three large, randomized, phase 3 clinical trials have shown its efficacy versus standard-dose allopurinol (300 mg/d). In the Febuxostat Versus Allopurinol Controlled Trial, 74% of the patients who received febuxostat, 80 mg/d, achieved a serum urate level lower than 6 mg/dL, compared with 36% of those who received allopurinol at 300 mg/d, by week 52.12
A second phase 3 study, the Allopurinol- and Placebo-Controlled Efficacy Study of Febuxostat trial, had similar results13; 76% of the febuxostat 80 mg/d population achieved the target goal of lower than 6 mg/dL versus 41% in the allopurinol 300 mg/d group. Higher dosages of febuxostat were even more effective; 94% of patients in a 240 mg/d arm and 87% in a 120 mg/d arm achieved the target serum urate level. (Dosages higher than 80 mg/d have not been FDA-approved; the 120 mg/d dosage is approved in Europe.)
A third phase 3 study, as yet published only in abstract form, again showed that 80 mg/d of febuxostat is superior to 300 mg/d of allopurinol (serum urate level lower than 6 mg/dL achieved by 67% and 42% of patients, respectively) and that febuxostat, 40 mg/d, and allopurinol, 300 mg/d, are equally efficacious.14 These trials also supported the notion that febuxostat is effective and safe in patients who have mild to moderate renal insufficiency (estimated glomerular filtration rate, 30 to 90 mL/min).
Because lowering serum urate levels transiently predisposes to gouty attacks, patients starting any urate-lowering therapy should receive prophylaxis for 3 to 6 months with colchicine (0.6 mg qd or bid) or, rarely, with an alternative anti-inflammatory agent. In fact, febuxostat appears to convey a greater early risk of attack than allopurinol, probably reflecting its greater urate-lowering capacity.
Although febuxostat is more potent than allopurinol on a per-molar basis and has generally outperformed allopurinol in clinical studies, it is not necessarily the superior drug. Note that virtually all the studies of febuxostat have used allopurinol as a comparator at 300 mg/d, but the latter agent is FDA-approved at up to 800 mg/d. Whether allopurinol at these higher dosages can outperform febuxostat has not been studied. However, the need to titrate allopurinol may result in a longer time to reach target serum urate levels relative to febuxostat.
Febuxostat safety and efficacy have not been assessed in patients with severe renal failure or in those who are receiving dialysis. However, clinical practice has so far supported the notion that patients with allopurinol allergies usually may take febuxostat without showing cross-reactivity.
Uricase replacement
Hyperuricemia in humans primarily is a consequence of the absence of a functional uricase enzyme. Therefore, repleting the missing enzyme via intravenous infusion is a third potential avenue for lowering uric acid. (We have reviewed the possible reasons why among all mammals, primates have a missense mutation in the uricase gene.15)
To this end, rasburicase (recombinant Aspergillus flavus uricase) was developed for the prevention or management of hyperuricemia in pediatric tumor lysis syndrome. Case reports have demonstrated the ability of rasburicase to lower serum urate levels in patients with refractory gout; however, rasburicase is highly immunogenic, and its use for more than 1 or 2 administrations may result in both the generation of neutralizing antibodies and severe hypersensitivity reactions.
Pegloticase is a recombinant, pegylated mammalian (porcine/baboon hybrid) uricase that was developed in an attempt to overcome the immune limitations of rasburicase and permit extended uricase supplementation. Pegylation (the alteration of proteins by the addition of several chains of polyethylene glycol) is an established strategy to mask the antigenicity of foreign proteins and permit their administration as therapeutic agents.
Pegloticase has been tested in replicated 6-month phase 3 trials; FDA approval currently is pending. Both trials enrolled patients who had severe gout; most had tophi and more than 6 flares per year. Most also had contraindications to allopurinol. Patients were randomized to receive 8 mg of pegloticase intravenously every 2 (n = 85) or 4 (n = 84) weeks or placebo (n = 43).
By the end of the study, 43% of patients in the every-2-week arms and 34% of the patients in the every-4-week arms reached the primary end point of a serum uric acid level lower than 6 mg/dL, compared with 0% of patients in the placebo group.16 This somewhat low rate of achieving the primary end point was the result, in part, of a fairly high dropout rate because of adverse events and, in part, because of a loss of efficacy as the study continued. However, patients who responded to the drug persistently did extremely well. Of the 85 patients in the 8-mg every-2-week arm, 36 maintained serum urate levels lower than 2 mg/d throughout the study. These impressive responses suggest that pegloticase may be particularly useful for the resolution of tophi and depletion of the total body urate burden.
Patients who experienced only a transient response to pegloticase had a dramatic and rapid drop in serum urate levels after the first infusion, equivalent to those of the persistent responders. By the end of the study, however, their serum urate levels rose to those of the placebo group. The loss of efficacy in the transient responders appeared to be mainly the result of the production of antipegloticase antibodies; transient responders had 70-fold higher antibody titers than persistent responders, with a concomitant absence of drug in serum because of clearance.17 Of note, most of the antigenicity was directed not to the uricase itself but to the polyethylene glycol that was added to reduce immunoreactivity. Decreasing antigenicity undoubtedly will be a key to improving outcomes and reducing the adverse effects of uricase supplementation as a matter of future development.
One question about pegylated uricase is where it might fit into a routine gout treatment regimen, assuming it obtains FDA approval. Pegylated uricase clearly will be an expensive drug. In addition, this agent is likely to have toxicities and as an intravenous therapy it will be inconvenient compared with oral medications. For these and other reasons, FDA approval is being sought only for patients for whom aggressive treatment with standard agents has not been successful. Nonetheless, pegylated uricase may be particularly useful in patients who have a high total body urate burden, including those with tophaceous gout.
Urate lowering in the absence of gout
Accumulating in the literature is the suggestion that hyperuricemia may carry its own set of undesirable consequences, even in patients who do not have gout. It has long been appreciated that patients with hyperuricemia frequently also have cardiovascular disease (CVD), but this association was considered to be the result of common risk factors rather than cause and effect.
However, several recent large epidemiological studies have suggested that hyperuricemia may actually be an independent risk factor for CVD. Animal models and human interventional trials also suggest that hyperuricemia contributes directly to the development of hypertension, CVD, and even insulin resistance and the metabolic syndrome. Given the increasing ability to alter uric acid levels, some physicians have asked whether hyperuricemia deserves treatment even in patients who do not have gout. However, most experts would probably agree that the available evidence has not yet achieved sufficient certainty to support the indiscriminate lowering of serum urate levels.18
CONCLUSIONS
Although the previously developed treatments for patients with gout have considerable efficacy, new therapeutic agents have been needed for some time. Febuxostat, FDA-approved colchicine, and anakinra are available immediately, and other agents are likely to follow. Gout management probably will enter a state of some flux as clinicians strive to understand how best to use both the established and the newer agents. There can be little doubt, however, that the new era of gout management will allow clinicians to offer their patients more effective and safer treatment options. In particular, the ability to reduce or resolve tophi, currently limited, probably is one area in which the new agents will demonstrate significant superiority.
References:
References1. Choi H. Epidemiology of crystal arthropathy. Rheum Dis Clin North Am. 2006;32:255-273, v.
2. Terkeltaub R, Furst DE, Bennett K, et al. Colchicine efficacy assessed by time to 50% reduction of pain is comparable in low dose and high dose regimens: secondary analyses of the AGREE trial: abstract 1103. Arthritis Rheum. 2009;60(suppl):S413.
3. Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237-241.
4. So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28.
5. Terkeltaub R, Sundy JS, Schumacher HR, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009;68:1613-1617.
6. Schumacher HR Jr, Sundy JS, Terkeltaub R, et al. Placebo-controlled study of rilonacept for gout flare prophylaxis during initiation of urate-lowering therapy. Arthritis Rheum. 2009;60(10 suppl):S410.
7. So A, De Meulemeester M, Shamim T, et al. Canakinumab (ACZ885) vs triamcinolone acetonide for treatment of acute flares and prevention of recurrent flares in gouty arthritis patients refractory to or contraindicated to NSAIDs and/or colchicine. Arthritis Rheum. 2009;60:3860. Presented at: ACR/ARHP Annual Scientific Meeting presentation LB4; October 20, 2009; Philadelphia.
8. Reinders MK, van Roon EN, Jansen TL, et al. Efficacy and tolerability of urate-lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinol. Ann Rheum Dis. 2009;68:51-56.
9. Lasko B, Sheedy B, Hingorani V, et al. RDEA594, a novel uricosuric agent, significantly reduced serum urate levels and was well tolerated in a phase 2a pilot study in hyperuricemic gout patients. Arthritis Rheum. 2009; 60(suppl):S413. Presented at: ACR/ARHP Annual Scientific Meeting presentation 1105; October 19, 2009; Philadelphia.
10. Beara-Lasic L, Pillinger MH, Goldfarb DS. Advances in the management of gout: critical appraisal of febuxostat in the control of hyperuricemia. Int J Nephrol Renovasc Dis. 2010;3:1-10.
11. Sarawate CA, Brewer KK, Yang W, et al. Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin Proc. 2006;81:925-934.
12. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450-2461.
13. Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540-1548.
14. Becker M, Schumacher HR Jr, Espinoza L, et al. A phase 3 randomized, controlled, multicenter, double-blind trial (RCT) comparing efficacy and safety of daily febuxostat (FEB) and allopurinol (ALLO) in subjects with gout. Arthritis Rheum. 2008;58:4029.
15. Pillinger MH, Rosenthal P, Abeles AM. Hyperuricemia and gout: new insights into pathogenesis and treatment. Bull NYU Hosp Jt Dis. 2007;65:215-221.
16. Sundy JS, Becker BH, Edwards MA, et al. Efficacy and safety of intravenous (IV) pegloticase (PGL) in subjects with treatment failure gout (TFG): phase 3 results from GOUT1 and GOUT2. Arthritis Rheum. 2008;58 (suppl):S400.
17. Becker MA, Treadwell EL, Baraf HS, et al. Immunoreactivity and clinical response to pegloticase (PGL): pooled data from GOUT1 and GOUT2, PGL phase 3 randomized, double blind, placebo-controlled trials. Arthritis Rheum. 2008;58(suppl):S880. Abstract 1945.
18. Keenan RT, Pillinger MH. Hyperuricemia, gout, and cardiovascular disease-an important“muddle.” Bull NYU Hosp Joint Dis. 2009;67:285-290.




