Article
Pfizer COVID-19 Vaccine Potentially Associated with Severe Allergic Reactions
Author(s):
The UK’s regulatory agency is advising those with a history of severe allergies not to get vaccinated pending investigations of 2 severe adverse events.
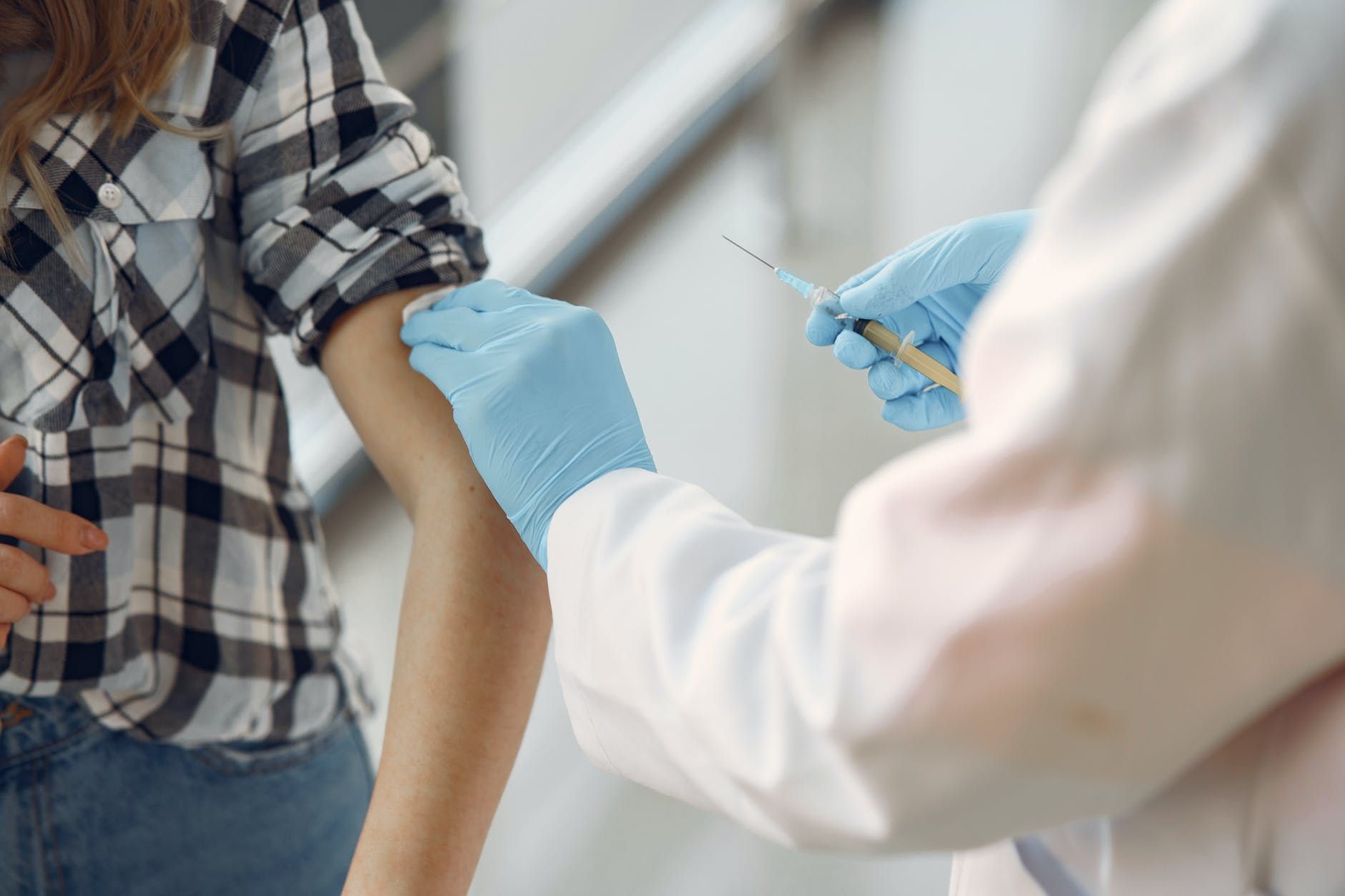
On the first day of public roll-out of Pfizer-BioNTech’s coronavirus disease (COVID-19) vaccine in the UK, 2 National Health Service (NHS) workers developed anaphylactoid reactions associated with receiving the vaccine.
The reports have prompted Britain’s Medicines and Healthcare products Regulatory Agency (MHRA) to advise those with a history of severe allergies against vaccination.
“As is common with new vaccines the MHRA (regulator) have advised on a precautionary basis that people with a significant history of allergic reactions do not receive this vaccination, after two people with a history of significant allergic reactions responded adversely yesterday,” said NHS medical director Stephen Powis.
Nonetheless, he stated that both individuals are recovering well.
With the support of Pfizer and BioNTech, the MHRA will conduct an investigation to gather more information regarding these cases.
MHRA Chief Executive June Raine indicated that allergic reactions to the vaccine were not initially reported in their clinical trials.
Briefing documents released by the US Food and Drug Administration (FDA) indicated that 0.63% of participants in the vaccine group and 0.51% in the placebo group reported possible allergic reactions in the trials, numbers which Peter Openshaw, Professor of Experimental Medicine at Imperial College London, acknowledged to be very small.
“The fact that we know so soon about these two allergic reactions and that the regulator has acted on this to issue precautionary advice shows that this monitoring system is working well,” Openshaw said.
About Pfizer’s Phase 3 Study - The BNT162b2 Vaccine
Data from the trial currently indicates that the 2-dose regimen was 95% effective overall (95% CI, 90.3–97.6), with a greater efficacy seen in those aged 16-55 years old (95.6%; 95% CI, 89.4-98.6) than those >55 years old (93.7%; 95% CI, 80.6-98.8).
As for those considered “at-risk” for COVID-19, defined as having ≥1 Cahrlson comorbidity index item or body mass index (BMI) of ≥30, the vaccine was associated with 95.4% efficacy (95% CI, 87.8-98.8).
“At-risk” patients aged ≥65 years were associated with a 91.7% vaccine efficacy (95% CI, 44.2-99.8), though the investigators acknowledge this population was too small to make an absolute determination.
The overall evaluable population—or those who received both vaccine doses—was 37,088 patients. Of this population, 81.9% were white 26.2% were Hispanic/Latino, 9.8% were African American, 4.4% were Asian, <3% were from other racial groups. Furthermore, 49.4% were female, and 21.4% were ≥65 years old.
Comorbidities were reported in 46.2% of these patients—which included obesity (35.1%), diabetes (8.4%), and pulmonary diseases (7.8%).
Safety Profile
The investigators observed adverse events among a greater proportion of vaccine recipients than among those who received placebo.
Injection site pain was the most frequently reported solicited local reaction among participants ≥18 years old. This was even more frequent after the second dose and was generally considered to be moderate.
Solicited systemic events—which was reported with greater frequency in younger participants—included fever, fatigue, headache, chills, vomiting, diarrhea, and new or worsened muscle/join pain. Similarly, these events were reported more frequently following second dose injection.
Furthermore, among all age groups, the most common events were fatigue, headache and new or worsened muscle pain.
In terms of unsolicited non-serious adverse events, the investigators reported an imbalance of lymphadenopathy in the vaccination group (n = 64) —compared with the placebo (n = 4). There were also 4 vaccinated cases of Bell’s palsy, though the investigators indicated it is not yet clear whether there is a causal relationship with the vaccine.
Serious adverse events in the vaccine group related to the vaccine included shoulder injury, ventricular arrhythmia, and lymphadenopathy.
Furthermore, 6 patients died overall, 2 of whom were vaccine recipients.
And finally, there were no reported safety concerns by participant age, race, ethnicity, medical comorbidities, or prior infection.
Tomorrow, the FDA will consider the efficacy and safety data in its review of the companies’ Emergency Use Authorization (EUA) application.





