Article
Phase 1/2 Trial Reports Positive Initial Clinical Data of VRDN-001 in Thyroid Eye Disease
Author(s):
Significant rapid improvement were observed in both signs and symptoms of thyroid eye disease at week 6 after 2 infusions of 10mg/kg VRDN-001.
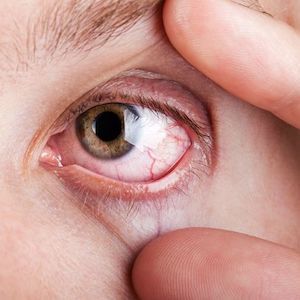
This week, Viridian Therapeutics, Inc reported positive initial clinical data from the ongoing Phase 1/2 clinical trial of the anti-IGF-1R antibody VRDN-001 in patients with active thyroid eye disease (TED).
The double-blind, placebo-controlled Phase 1/2 trial is evaluating two infusions of VRDN-001 administered intravenously, with efficacy measurements including proptosis, Clinical Activity Score (CAS), and diplopia.
Data suggest significant and rapid improvements in both signs and symptoms of TED at week 6 after two infusions of 10 mg/kg VRDN-001. The findings report proptosis response (≥2mm reduction) was achieved by 83% of patients, with a 2.3 mm mean reduction from baseline.
Moreover, the Clinical Activity Score of 0 or 1 was achieved by 83% of patients with a mean reduction of 4.3 points from baseline and complete resolution of diplopia was achieved by 75% of patients with diplopia at baseline.
“TED is a severe, debilitating eye disease that threatens patients’ vision and overall quality of life,” said Raymond Douglas, MD, PhD, Director of Orbital and Thyroid Eye Disease Program, Cedars-Sinai Medical Center and investigators of VRDN-001 trial. “In this trial, rapid and significant improvements in a broad set of efficacy measures suggests that VRDN-001 may have a differentiated profile that could offer meaningful benefits to patients.”
The ongoing trial is evaluating two infusions of VRDN-001 3 weeks apart with efficacy measured 6 weeks following the first dose. Each dose is evaluated in a cohort of 8 patients, randomized so that 6 patients receive VRDN-001 and 2 patients receive placebo.
A second cohort is evaluating a dose of 20 mg/kg with results expected in the fourth quarter of 2022. A third cohort will evaluate a dose of 3 mg/kg with data expected in the fourth quarter of 2022.
The agent was well-tolerated by all patients treated at the 10mg/kg dose, with no reported serious adverse events (SAEs), no patient discontinuations, and no hyperglycemia or infusion reactions of August 2022. They reported two cases of mild muscle spasm which did not require intervention, as well as a single report of “ringing in the ears” which was later resolved.
In addition, the ongoing second cohort at 20 mg/kg has reported no adverse events of hyperglycemia, muscle spasm, hearing impairment, infusion reactions, or any serious adverse events as of August 9, 2022.
“These data exceeded our expectations and compare very favorably to Tepezza, the only approved therapy in TED,” said Barrett Katz, MD, MBA, Chief Medical Officer at Viridan in a statement. “We plan to accelerate the start of our Phase 3 THRIVE program, now starting later this year, in a broad TED population of both active and chronic disease. We believe VRDN-001 could show a more rapid onset of action and afford a shorter course of treatment with the potential for a differentiated profile versus other TED therapies, due to its full antagonism of IGF-1R.”





