News
Article
Prophylaxis Increases Risk of Adverse Events in Patients Initiating Allopurinol for Gout
Author(s):
In patients initiating allopurinal, adverse events were more common in those receiving concomitant colchicine or nonsteroidal anti-inflammatory drugs compared to those without prophylaxis.
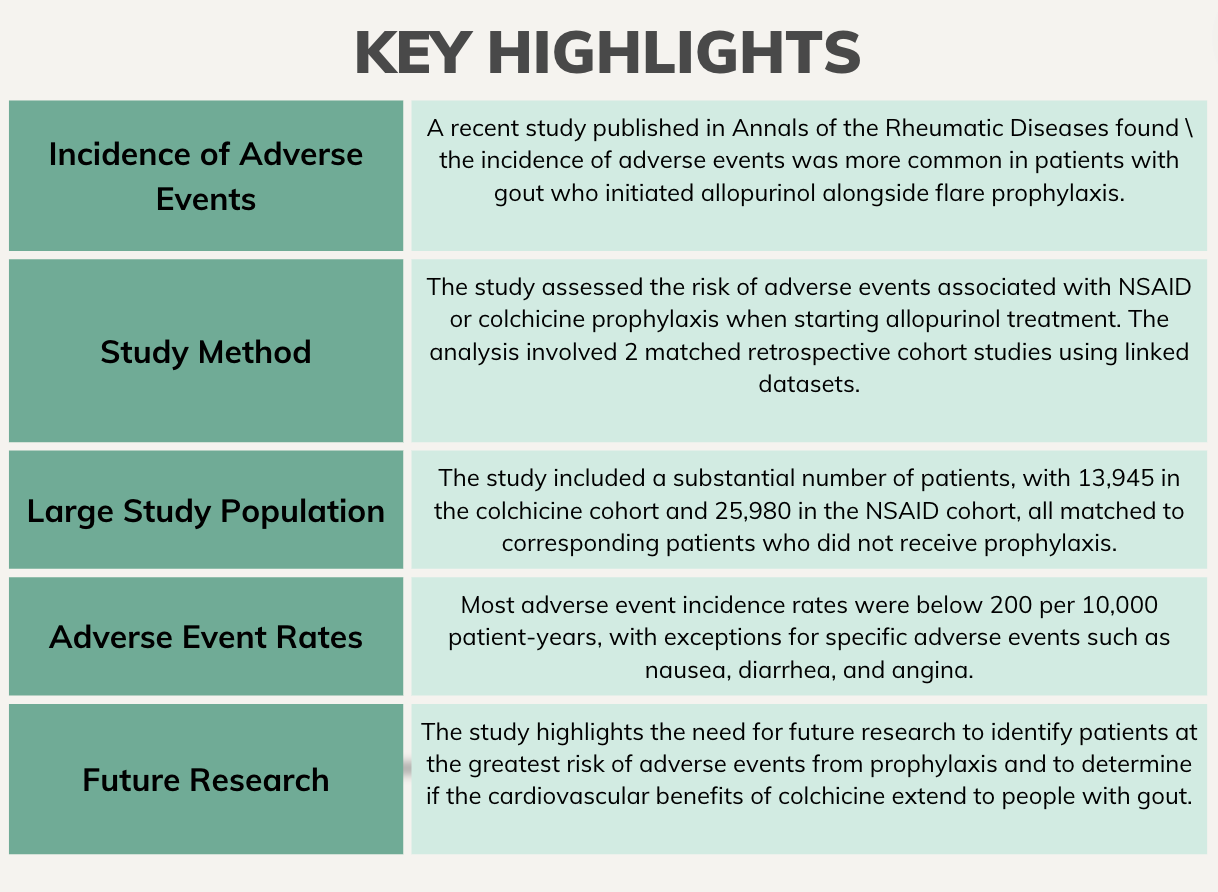
The incidence of adverse events were more common in patients with gout initiating allopurinol in conjunction with flare prophylaxis, according to a study published in Annals of the Rheumatic Diseases.1 However, serious events were uncommon, offering reassurance for clinicians and patients during the shared decision-making process.
“The long-term management of gout involves taking urate-lowering therapy (ULT), most commonly allopurinol, to reduce serum urate levels which, over several months, leads to gradual dissolution of monosodium urate (MSU) crystals and cessation of flares,” wrote lead investigator Edward Roddy, PhD, professor of rheumatology, Honorary Consultant Rheumatologist, Keele University, Keele, UK, and colleagues. “Although the aim of ULT is to prevent flares, initiation or increasing the dose of ULT often triggers a gout flare, which can lead to ULT being stopped as patients and practitioners may believe that it has worsened the gout.”
Therefore, guidelines recommend prescribing concomitant prophylactic medications for several months during the initiation of ULT to prevent and reduce gout flare. Colchicine, specifically, is the first-line recommended drug throughout the initiation period.2
Investigators assessed the risk of adverse events linked to nonsteroidal anti-inflammatory drug (NSAID) or colchicine prophylaxis when beginning allopurinol treatment using 2 matched retrospective cohort studies in linked Hospital Episode Statistics and UK Clinical Practice Research Datalink (CPRD) datasets. Adult patients with gout initiating allopurinol with either NSAID or colchicine prophylaxis were compared with patients initiating the drug without prophylaxis. Patients were individually matched by sex, age, and propensity to receive the appropriate prophylaxis. The link between prophylaxis and specified adverse events were evaluated using weighted Cox proportional hazards models.
A total of 13,945 patients in the colchicine cohort were matched to 13945 patients with no prophylaxis, and 25,980 patients in the NSAID cohort were matched to 25,980 with no prophylaxis.
Adverse event incidence rates were <200/10,000 patient-years, with the exception of nausea (208.1; 95% confidence interval [CI] 165.4 to 261.7) and diarrhea (784.4; 95% CI 694.0 to 886.5) in the colchicine group and angina (466.6; 95% CI 417.2 to 521.8) in the NSAID group.
Adverse events were more common in patients receiving colchicine compared with no prophylaxis, including diarrhea (hazard ratio [HR] 2.22; 95% CI 1.83 to 2.69), neuropathy (4.75; 95% CI 1.20 to 18.76), bone marrow suppression (3.29; 95% CI 1.43 to 7.58), myalgia (2.64; 95% CI 1.45 to 4.81), and myocardial infarction (MI) (1.55; 95% CI 1.10, 2.17). However, nausea and vomiting were comparable (1.34; 95% CI .97 to 1.85).
Patients receiving NSAID prophylaxis were more likely to experience angina (1.60; 95% CI 1.37 to 1.86), myocardial infarction (1.89; 95% CI 1.44 to 2.48), acute kidney injury (1.56; 95% CI 1.20 to 2.03), and peptic ulcer disease (1.67; 95% CI 1.14 to 2.44) compared with those without.
Investigators noted the large sample size coupled with data linked to hospital records over a 20-year span as strengths of the study. However, gout was diagnosed through a clinical diagnosis as opposed to classification criteria. Additionally, only adverse events severe enough to require a consultation or hospitalization were included in the study. Therefore, milder events may have been missed. The observational nature of the study created the possibility of misclassification and investigators could not make causal inferences.
“Future research is needed to determine which patients are at greatest risk of adverse events from prophylaxis and whether the cardiovascular benefits of colchicine reported in randomized controlled trials of people at high risk of cardiovascular events because of a prior history of coronary heart disease also apply to people with gout,” investigators concluded. “Our findings will provide much-needed information about the safety of flare prophylaxis that can inform treatment decisions and the choice between colchicine or NSAID for prophylaxis when initiating allopurinol, directly benefiting people with gout and their clinicians.”
References
- Roddy E, Bajpai R, Forrester H, et al. Safety of colchicine and NSAID prophylaxis when initiating urate-lowering therapy for gout: propensity score-matched cohort studies in the UK Clinical Practice Research Datalink [published online ahead of print, 2023 Oct 3]. Ann Rheum Dis. 2023;ard-2023-224154. doi:10.1136/ard-2023-224154
- Richette P, Doherty M, Pascual E, et al. Updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42. doi:10.1136/annrheumdis-2016-209707




