Article
Psoriasis Drug Taltz Provides Long-Term Relief
Author(s):
After the FDA’s March approval of ixekizumab (Taltz/ Eli Lilly and Company), researchers recently confirmed the psoriasis treatment was beneficial even after 60 weeks.
After the FDA’s March approval of ixekizumab (Taltz/ Eli Lilly and Company), researchers recently confirmed the psoriasis treatment was beneficial even after 60 weeks.
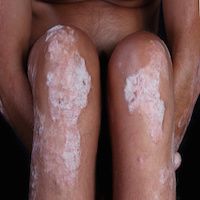
Researchers found that Taltz previously showed “unprecedented” effects on the skin condition, trumping standard medication for moderate-to-severe psoriasis.
The findings, published in the New England Journal of Medicine, are from a study that included nearly 4,000 patients who participated in three trials: the first trial tested ixekizumab against a placebo, but the second and third trials compared ixekizumab to placebo and another psoriasis drug (Enbrel) for the first 12 weeks, then just placebo for the remainder of the study.
Following the first 12-week period, patients were allowed to take the drug either once a month or every 12 weeks.
Study results showed that after 60weeks, about 80% of the patients experienced at least a 75% improvement in their symptoms, ranging from thick, itchy, and painful scaly patches on the skin to aching joint damage or fatigue.
According to Joel Gelfand, MD, Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, the injectable drug specifically targets an inflammatory protein called IL-17 — another “revolution” in psoriasis treatment.
However, researchers and clinicians are urged to continue monitoring the drug’s long-term effects.
The drug’s recommended dose is one injection “every couple of weeks” for the first three months, then switching to every four weeks.



