Article
Recognition of Methotrexate Toxicity Key for Patient Treatment
Author(s):
For more than 70 years methotrexate has been a popular tool in the prescription toolkit of doctors around the world. However, a recent case report by a team from the United Kingdom showed how important it is for prescribing doctors to also keep a close eye on some of the well-known adverse events associated with the medication.
For more than 70 years methotrexate has been a popular tool in the prescription toolkit of doctors around the world. However, a recent case report by a team from the United Kingdom showed how important it is for prescribing doctors to also keep a close eye on some of the well-known adverse events associated with the medication.
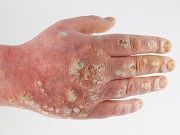
One of the most serious potential consequences of taking methotrexate is hepatoxicity and myelosuppression. The study looked specifically at another potential side effect in methotrexate-induced cutaneous ulceration in a patient with chronic plaque psoriasis which occurred during their treatment.
The patient in the case study was an 83-year-old woman with “long-standing severe chronic plaque psoriasis,” who had a 3-week history of oral ulceration and a 2-week flare of psoriasis. The woman also reported having taken methotrexate for more than 10 years with her dosage increased to a maximum of 25 mg taken once every week. She was also taking 5 mg of folic acid once a week on a different day.
In addition to psoriasis the patient also reported suffering from hypertension and congestive heart failure and had been hospitalized for cardiac failure. The authors reported observing oral erosions of the buccal mucosa as well as “large, painful, inflamed and ulcerated psoriatic plaques each with a prominent tender erythematous weepy inflamed edge.”
Study authors reported that by taking the patient off of methotrexate and “appropriate skin care,” resulted in “rapid healing of the ulceration and the agent was later safely reintroduced for the ongoing management of the patient’s chronic plaque psoriasis.” The treatment reportedly took 2 weeks of full withdrawal from the methotrexate before being reintroduced at a lower dose of 10 mg with concomitant folic acid supplementation of 5 mg daily for the six days of the week methotrexate was not taken.
Research as part of the study indicated that some of the most common “triggers” of the ulceration included accidental overdose, or the introduction of an “interacting agent.” The authors added, “Early recognition, prompt cessation of methotrexate and appropriate treatment minimizes morbidity.”
The authors noted in their report that, “Perhaps surprisingly, most cases of methotrexate-induced ulceration have been reported in patients with psoriasis on low-dose treatment rather than in high-dose oncology regimens.” They added, “It is postulated that the increased proliferation of keratinocytes within psoriatic plaques makes them particularly vulnerable to the effects of folate-antagonism.”
Part of the reason the authors said treatment of the ulceration was so successful so quickly was their ability to recognize the symptoms in a timely manner. “Early recognition of cutaneous findings as a marker of methotrexate toxicity in the reported case allowed prompt cessation of the drug avoiding serious morbidity.” They added, “It is vital that dermatologists are confident in the recognition and management cutaneous and non-cutaneous manifestations of methotrexate toxicity in their own patients and those of their specialties.





