Article
Rheumatoid Arthritis: Current Diagnosis and Treatment Practices
New rheumatoid arthritis (RA) classification criteria were published in 2010. Although classification criteria are intended primarily for clinical trials, they may be used as diagnostic aids in clinical practice and may affect treatment strategies.
ABSTRACT: New rheumatoid arthritis (RA) classification criteria were published in 2010. Although classification criteria are intended primarily for clinical trials, they may be used as diagnostic aids in clinical practice and may affect treatment strategies. Early achievement and maintenance of remission results in improved functional and radiographic outcomes. Treatment strategies that focus on “treatment to target” have been shown to optimize outcomes in RA. Corticosteroids are used frequently in early arthritis in conjunction with disease-modifying therapies. Currently approved tumor necrosis factor α inhibitors have been used successfully in conjunction with other disease-modifying antirheumatic drugs and more often are used as combination therapy. Newer agents in development have been shown to be effective.
___________________________________________________________________________________
How Patients Are ClassifiedTreatment Strategies
Corticosteroids
Conventional disease-modifying drugs
on page 2: Biologic agents
Adverse Events
Targets in Development
Rheumatoid arthritis (RA), estimated to affect 1.3 million adults in the United States, is the most common of the inflammatory arthritides.1 The pathogenesis is characterized by acute and chronic inflammatory changes to the synovium that over the long term result in joint damage, most of which has been recognized to occur early in the disease course.2,3
New RA classification criteria published in 2010 were developed by a joint working group from the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR). Given the observed limitations of the 1987 criteria for detecting disease at the earliest stages and a shift in focus of disease management to these stages, the new approach sought to identify patients who have undifferentiated inflammatory arthritis factors associated with persistent or erosive disease or both.4-6 Compared with the 1987 criteria, the 2010 criteria have been shown to classify more patients with RA and at an earlier stage of disease, with a sensitivity and specificity of 0.71 and 0.65, respectively, using persistent arthritis as the outcome.6
Although classification criteria are intended for use in the identification of persons with RA primarily for enrollment in clinical trials, they may be used as diagnostic aids in clinical practice6 and may affect treatment strategies. With recognition that clinical outcomes are improved with earlier treatment, identifying patients for whom the risk of structural damage is high has become quite important in guiding management with disease-modifying therapies.4 Here we provide an overview of the latest RA diagnosis and treatment practices.
HOW PATIENTS ARE CLASSIFIED
The new criteria classify patients as having definite RA using a score-based algorithm applied to eligible patients-those for whom there is (1) evidence of clinical synovitis in at least 1 joint and (2) no alternative explanation for synovitis.4 With the emphasis on identification of patients before the development of radiographic erosions, the new classification criteria do not include the presence of these erosions.
Anti–cyclic citrullinated peptide (anti-CCP) antibodies are included in the classification criteria. This has both diagnostic and prognostic advantages because of the high specificity of the anti-CCP antibodies and the association with radiological progression of erosive changes and a more aggressive disease course.7-9
The identification of clinical synovitis as a prerequisite for application of the classification criteria underscores the importance of the physical examination. In cases in which examination findings are negative, imaging studies may play a role in identifying patients. The new criteria provide for classifying patients who have erosive disease typical of RA with a compatible history as having definite RA.4
Conventional radiographs remain a useful aid in diagnosis and in management of patients. Compared with advanced imaging modalities, they have the advantages of ready availability and lower cost, but they are insensitive for detection of the earliest changes of bone erosions. MRI and ultrasonography allow for detection of early inflammatory changes that involve the soft tissue and early stages of bone erosion, allowing for direct visualization of early inflammatory and destructive changes.10
In the clinic, conventional radiographs remain the initial imaging modality of choice, but when the results are negative, advanced imaging may be considered for evaluation of subclinical synovitis and detection of clinical improvement and regression of synovitis.11 The use of advanced imaging for assessment of disease activity probably will evolve over time, with the current emphasis on initiating treatment before development of joint damage and erosive changes and with development of better methods for detecting subclinical disease activity, which may still progress to joint damage.
TREATMENT STRATEGIES
Early achievement and maintenance of remission results in improved functional and radiographic outcomes.12-15 Treatment strategies that focus on “treatment to target”-measuring disease activity and adjusting therapy with the goal of remission or low disease activity-have been shown to optimize outcomes in RA when compared with a routine approach.16-18
Composite indices, which use changes of a group of variables rather than individual signs and symptoms, have been shown to mirror disease activity more closely than individual variables.19 Composite disease activity scores now in use-including the Disease Activity Score (DAS), the 28-joint Disease Activity Score (DAS 28), the Simplified Disease Activity Index (SDAI), the Clinical Disease Activity Index (CDAI), and the Routine Assessment of Patient Index Data 3 (RAPID3)-have been shown to be predictive of future disease activity.20 They have been used in clinical trials for measures of disease outcome21 and are used in routine clinical practice to guide treatment decisions.
DAS 28 erythrocyte sedimentation rate score–driven therapy in patients with recent-onset RA was shown to result in more patients achieving remission without disability and radiographic progression than routine care.18 SDAI and CDAI definitions of clinical remission have been shown to be more restrictive than that of the DAS 28.22 Using Doppler ultrasonography as the gold standard for detection of synovitis, the SDAI was shown to be superior to the DAS 28 in the definition of clinical remission23; in one study, the SDAI was shown to be a predictor for a change in treatment when compared with the DAS 28 and DAS.24
The RAPID3 uses patient-reported measures only (physical function, pain, and patient global estimate) for assessment and monitoring. This index has been shown to correlate with the DAS 28 and CDAI in clinical trials and in clinical care.25,26 The RAPID3 has the advantage of the lack of requirement of a formal joint count, which allows for the assessment to be completed in a shorter period and may make it a more pragmatic option for use in the clinical setting.26Corticosteroids
These agents are used frequently in early arthritis in conjunction with the introduction of disease-modifying therapies because of their more immediate effects on reducing inflammatory symptoms. Low-dose prednisolone (7.5 mg/d) given in conjunction with disease-modifying therapy has been shown to result in higher rates of remission and better radiographic outcomes when used in patients with early RA.27-29
In the Behandel Strategien study, use of prednisone at an initial dosage of 60 mg/d was associated with improvement in functional outcome and less radiographic progression at 1 year of follow-up.14 Because of concerns about the adverse effects of corticosteroids, including fracture, risk of infection, and cardiovascular disease, they should be used for only a limited period and at the lowest effective dose.
Intermittent use of corticosteroids for management of disease flares at dosages of 15 mg/d or less for active synovitis has been associated with improved disease activity compared with placebo in patients with long-standing RA (disease duration longer than 2 years).30 Intra-articular corticosteroids also offer an effective strategy for management of disease flares in cases of monarticular or oligoarticular joint involvement.31Conventional disease-modifying drugs
Disease-modifying antirheumatic drugs (DMARDs)-methotrexate (MTX), hydroxychloroquine (HCQ), leflunomide, and sulfasalazine (SSZ)-are used widely in the early stages of the RA disease process.32 MTX remains the most frequently used DMARD.
Compared with single-drug therapy, the use of 2 or 3 DMARDs in combination results in better rates of remission induction, improved functional outcomes, and similar adverse event rates.13 However, a recently published Cochrane database systematic review that compared MTX monotherapy with MTX combined with nonbiologic DMARDs did not show a significant advantage of MTX combination therapy in DMARD-naive patients.33
A widely used therapeutic strategy is the initial use of MTX monotherapy for early RA with the addition after 6 months, if required, of SSZ or HCQ or a tumor necrosis factor α (TNF-α) inhibitor. In support of this approach, a recent 2-year double-blind trial of patients with early RA found no differences in mean DAS 28 scores during weeks 48 to 102 among patients randomized to etanercept or triple DMARD therapy (MTX, SSZ, and HCQ) regardless of whether immediate combination treatment was used or MTX monotherapy was initiated and step-up treatment was given.34
At 6 months, immediate combination therapy (MTX and etanercept vs triple combination therapy) was more effective than MTX monotherapy, but there was no significant difference between the groups at 2-year follow-up. Although there was no demonstrated difference in clinical outcomes between the groups, preliminary analysis of the radiographic outcomes suggests that treatment with etanercept and MTX resulted in statistically significant radiographic benefit compared with triple therapy. The final results are not yet published.
Leflunomide monotherapy has been shown to have efficacy similar to that of MTX (the dose of MTX did not exceed 15 mg in trials, although doses in excess of this are used frequently in practice).35 Leflunomide monotherapy also has been used in combination with MTX in patients who have active disease while receiving stable doses.
Biologic agentsAdverse EventsTargets in Development
Biologic agents
Efforts to develop more effective treatment strategies for patients with RA hinge on understanding the role of inflammatory mediators in the disease process. Interleukin (IL)-1, IL-6, and TNF-α inhibitors have been identified as key cytokines that drive the inflammatory process in RA.36 Additional agents target B-cell depletion and T-cell costimulation.37
TNF-α inhibitors currently FDA-approved are infliximab, etanercept, adalimumab, golimumab, and certolizumab. Of these agents, adalimumab, certolizumab, etanercept, and golimumab are approved as monotherapy for RA, but they also have been used successfully in conjunction with other DMARDs and more often are used as combination therapy.38Infliximab. This is a chimeric human-mouse anti–TNF-α monoclonal antibody.39 Infliximab used in combination with MTX in patients with early RA has been shown to inhibit progression of structural damage and radiographic progression.40 Infliximab is the only TNF-α inhibitor currently in use that is administered as an infusion.
Etanercept. This recombinant human TNF receptor-Fc soluble fusion protein has been shown to reduce disease activity in patients with RA.41 The use of etanercept in combination with MTX in patients with early, moderate to severe RA showed outcomes superior to those with MTX monotherapy with regard to clinical remission at 1 year (50% compared with 28%) and radiographic nonprogression (80% compared with 59%).35Adalimumab. This is a recombinant fully human IgG1 monoclonal antibody. Combination therapy with MTX and adalimumab was superior to adalimumab monotherapy in improving signs and symptoms in patients with early, aggressive RA at 1 year (ACR 50 response was achieved in 62% and 41% of patients, respectively), effecting clinical remission and inhibition of radiographic progression.42Certolizumab and golimumab. These are the newer anti–TNF-α agents. Certolizumab pegol, a PEGylated TNF-α inhibitor, has been shown to be beneficial for patients with active RA; more patients achieved ACR 20 response than those who received placebo or they had less radiographic progression.43 Golimumab, a human monoclonal TNF antibody, has been studied for use in patients with ongoing disease activity who had been treated with a TNF-α inhibitor (but therapy was discontinued because of loss of efficacy, intolerance, or lack of accessibility).44 In clinical trials, ACR 20 response at week 14 was achieved in a larger number of treated patients than patients given placebo (up to 38% vs 18% of patients).44
In patients who do not respond to one TNF-α inhibitor, switching to an alternative TNF-α inhibitor may be attempted or use of a biologic agent with an alternative treatment target may be considered. The response rate to TNF-α inhibitor switching varies among patients.
Anakinra. This IL-1 receptor antagonist has been FDA-approved for use in patients with moderate to severe RA who have been unresponsive to initial DMARD therapy.43 A recent systematic review concluded that the degree of improvement noted was less than that seen in studies of other biologic agents,45 and this agent is used less widely than anti–TNF-α agents.
Abatacept. This selective T-cell costimulation modulator is another alternative to the TNF-α inhibitors. Patients with active RA who had an inadequate response to TNF-α inhibitor therapy were randomized to receive abatacept or placebo in addition to at least 1 DMARD; at 6 months, they had ACR 50 response rates of 20% compared with 4% and more patients in the abatacept group had clinically meaningful improvement in physical function.46Rituximab. This is a genetically engineered chimeric anti-CD20 antibody that targets peripheral B cells, which have been shown to play a role at various levels in the inflammatory pathway.47 Rituximab has been FDA-approved for management of moderate to severe RA given with MTX for patients for whom at least 1 TNF-α–blocking agent has resulted in an inadequate response.48 In current practice, patients in whom treatment with one TNF-α inhibitor was not successful often try an alternative TNF-α inhibitor before initiation of rituximab therapy.
TABLE 1
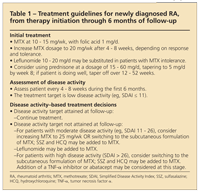
Treatment guidelines for newly diagnosed RA, from therapy initiation through 6 months of follow-up
In a prospective cohort study, patients who had previously discontinued at least 1 TNF-α inhibitor as a result of lack of efficacy showed clinically significant improvement with rituximab compared with switching to an alternative TNF-α inhibitor.49 The presence of the anti-CCP antibodies, rheumatoid factor positivity, and elevated IgG levels predict favorable response to rituximab in patients with refractory RA.50Tocilizumab. Overproduction of IL-6 has been shown to play a pathogenetic role in RA. Tocilizumab is a humanized anti–IL-6 receptor monoclonal antibody51 that when used with MTX is effective in inducing remission, sustaining clinical improvement, and improving function.52
Disease activity measures (the DAS 28 and CDAI) at 3 months are significantly related to disease activity at 1 year. Therefore, the 3-month mark serves as a good assessment point for reevaluation, because patients who do not achieve moderate or low levels of disease activity at 3 months after the start of DMARDs are unlikely to be in remission or have low disease activity states at 1 year. Alternative treatment strategies should be pursued both in patients with early RA who have not previously been exposed to DMARDs and in those with established disease and an inadequate response to MTX.20 Our approach to the management of early RA is summarized in Tables 1 and 2.
For the management of more established cases beyond the first year of disease, disease activity targets are reassessed at follow-up.53,54 If the target is attained, the DMARD regimen may be continued with tapering of prednisone for patients in remission. If there is sustained remission, consider de-escalation of therapy. For patients with low disease activity, the DMARD regimen should be continued. If the target is not attained, consider adding SSZ and HCQ and, in patients not receiving biologic agents, adding a TNF-α inhibitor or abatacept.
TABLE 2

Treatment guidelines for early RA, from 6 months to 1 year after treatment initiation
Preventive strategies are important in caring for patients with RA. Along with disease activity measures, they should be assessed periodically at follow-up.53,55-57ADVERSE EVENTS
Increased use of biologic agents has been accompanied by appropriate vigilance about possible adverse effects, including the risk of serious infections and malignancy. One early meta-analysis of harmful effects associated with anti–TNF-α antibody therapy use in RA showed an increased risk of serious infection and malignancy58; more recent meta-analyses with longer patient follow-up have suggested that the risk of serious infection is only minimally higher than at baseline for patients with RA who are receiving MTX therapy alone, and the overall risk of malignancy is not increased, other than the risk of nonmelanotic skin cancer.59
A recent meta-analysis that compared rates of serious infections for biologic agents with placebo did not reveal significant increases in the risk of serious infections in patients with RA during treatment with rituximab or abatacept.60 Although there is no direct evidence of a causal relationship, cases of progressive multifocal leukoencephalopathy associated with rituximab use have been reported.61
Recognition of the possibility of an increased risk of malignancy and infections is important in the clinician’s selection of treatment strategies. Such recognition also is important for informing patients in shared decision making.
TARGETS IN DEVELOPMENTOfatumumab. This anti-CD20 monoclonal antibody has been shown in phase 1 and 2 studies to be beneficial in the treatment of patients with active RA who have not been responsive to at least 1 DMARD.62 Phase 3 trials currently are under way.63,64Belimumab. This fully human anti–B-lymphocyte stimulator monoclonal antibody65 was FDA-approved in 2011 for treatment of patients with systemic lupus erythematosus. Studies of this agent in RA have been disappointing.66Atacicept. This recombinant fusion protein binds to the B-lymphocyte stimulator receptor (BLyS, CD257) and a proliferation-inducing ligand (APRIL, CD256), which are stimulators of B-cell maturation, proliferation, and survival.67,68 A study of atacicept in anti–TNF-α–naive patients with moderate to severe active RA and an inadequate response to MTX was done, but the results have not yet been published.69Tofacitinib. Janus kinase (JAK) 3, an intracellular molecule, plays a critical role in signal transduction of the receptors for IL-2, IL-4, IL-7, IL-19, IL-15, and IL-21 (related to lymphocyte activation, function, and proliferation).70 The oral JAK inhibitor tofacitinib has been shown in phase 2a trials to be effective in patients with active RA; ACR 20 response rates of up to 81% were achieved, and the agent was shown to result in improved pain and physical function compared with placebo.71,72R788. A recent 6-month, double-blind, placebo-controlled trial of an oral tyrosine kinase inhibitor (R788) in patients who had active RA in spite of receiving long-term MTX therapy showed ACR 20 response rates of up to 67% for patients receiving R788 compared with 35% for placebo patients.73 Diarrhea, upper respiratory tract infections, and elevated blood pressure were noted.
Problems/comments about this article? Please send feedback.
References:
References
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, part I. Arthritis Rheum. 2008;58:15-25.
2. Fuchs HA, Kaye JJ, Callahan LF, et al. Evidence of significant radiographic damage in rheumatoid arthritis within the first 2 years of disease. J Rheumatol. 1989;16:585-591.
3. van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35:26-34.
4. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative [published correction appears in Ann Rheum Dis. 2010;69:1892]. Ann Rheum Dis. 2010;69:1580-1588.
5. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315-324.
6. van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH. Classification of rheumatoid arthritis: comparison of the 1987 American College of Rheumatology criteria and the 2010 American College of Rheumatology/European League Against Rheumatism criteria. Arthritis Rheum. 2011;63:37-42.
7. Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2006;65:845-851.
8. Whiting PF, Smidt N, Sterne JA, et al. Systematic review: accuracy of anti-citrullinated Peptide antibodies for diagnosing rheumatoid arthritis. Ann Intern Med. 2010;152:456-464; W155-W166.
9. Lindqvist E, Eberhardt K, Bendtzen K, et al. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis. 2005;64:196-201.
10. Ãstergaard M, Pedersen SJ, Døhn UM. Imaging in rheumatoid arthritis-status and recent advances for magnetic resonance imaging, ultrasonography, computed tomography and conventional radiography. Best Pract Res Clin Rheumatol. 2008;22:1019-1044.
11. Scheel AK, Hermann KG, Ohrndorf S, et al. Prospective 7 year follow up imaging study comparing radiography, ultrasonography, and magnetic resonance imaging in rheumatoid arthritis finger joints. Ann Rheum Dis. 2006;65:595-600.
12. Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263-269.
13. Möttönen T, Hannonen P, Leirisalo-Repo M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet. 1999;353:1568-1573.
14. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381-3390.
15. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146:406-415.
16. Schoels M, Knevel R, Aletaha D, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search [published correction appears in Ann Rheum Dis. 2011;70:1519]. Ann Rheum Dis. 2010;69:638-643.
17. Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force [published corrections appear in Ann Rheum Dis. 2011;70:1349; Ann Rheum Dis. 2011;70:1519]. Ann Rheum Dis. 2010;69:631-637.
18. Soubrier M, Lukas C, Sibilia J, et al. Disease activity score-driven therapy versus routine care in patients with recent-onset active rheumatoid arthritis: data from the GUEPARD trial and ESPOIR cohort. Ann Rheum Dis. 2011;70:611-615.
19. Verhoeven AC, Boers M, van Der Linden S. Responsiveness of the core set, response criteria, and utilities in early rheumatoid arthritis. Ann Rheum Dis. 2000;59:966-974.
20. Aletaha D, Funovits J, Keystone EC, Smolen JS. Disease activity early in the course of treatment predicts response to therapy after one year in rheumatoid arthritis patients. Arthritis Rheum. 2007;56:3226-3235.
21. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67:1360-1364.
22. Burmester GR, Ferraccioli G, Flipo RM, et al. Clinical remission and/or minimal disease activity in patients receiving adalimumab treatment in a multinational, open-label, twelve-week study. Arthritis Rheum. 2008;59:32-41.
23. Balsa A, de Miguel E, Castillo C, et al. Superiority of SDAI over DAS-28 in assessment of remission in rheumatoid arthritis patients using power Doppler ultrasonography as a gold standard. Rheumatology (Oxford). 2010;49:683-690.
24. Soubrier M, Zerkak D, Gossec L, et al. Which variables best predict change in rheumatoid arthritis therapy in daily clinical practice? J Rheumatol. 2006;33:1243-1246.
25. Pincus T, Yazici Y, Bergman MJ. RAPID3, an index to assess and monitor patients with rheumatoid arthritis, without formal joint counts: similar results to DAS28 and CDAI in clinical trials and clinical care. Rheum Dis Clin North Am. 2009;35:773-778, viii.
26. Pincus T, Furer V, Keystone E, et al. RAPID3 (Routine Assessment of Patient Index Data 3) severity categories and response criteria: similar results to DAS28 (Disease Activity Score) and CDAI (Clinical Disease Activity Index) in the RAPID 1 (Rheumatoid Arthritis Prevention of Structural Damage) clinical trial of certolizumab pegol. Arthritis Care Res (Hoboken). 2011;63:1142-1149.
27. Svensson B, Boonen A, Albertsson K, et al. Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: a two-year randomized trial. Arthritis Rheum. 2005;52:3360-3370.
28. Kirwan JR, Bijlsma JW, Boers M, Shea BJ. Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev. 2007;(1):CD006356.
29. Möttönen TT, Hannonen PJ, Boers M. Combination DMARD therapy including corticosteroids in early rheumatoid arthritis. Clin Exp Rheumatol. 1999;17(6 suppl 18):S59-S65.
30. Gøtzsche PC, Johansen HK. Meta-analysis of short-term low dose prednisolone versus placebo and non-steroidal anti-inflammatory drugs in rheumatoid arthritis. BMJ. 1998;316:811-818.
31. Konai MS, Vilar Furtado RN, Dos Santos MF, Natour J. Monoarticular corticosteroid injection versus systemic administration in the treatment of rheumatoid arthritis patients: a randomized double-blind controlled study. Clin Exp Rheumatol. 2009;27:214-221.
32. Sizova L. Approaches to the treatment of early rheumatoid arthritis with disease-modifying antirheumatic drugs. Br J Clin Pharmacol. 2008;66:173-178.
33. Katchamart W, Trudeau J, Phumethum V, Bombardier C. Methotrexate monotherapy versus methotrexate combination therapy with non-biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis. Cochrane Database Syst Rev. 2010;(4):CD008495.
34. Moreland LW, O’Dell JR, Paulus H, et al. TEAR: Treatment of early aggressive RA; a randomized, double-blind, 2-year trial comparing immediate triple DMARD versus MTX plus etanercept to step-up from initial MTX monotherapy [abstract]. Arthritis Rheum. 2009;60(suppl 10):S1895.
35. Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375-382.
36. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907-916.
37. Nam JL, Winthrop KL, van Vollenhoven RF, et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA [published correction appears in Ann Rheum Dis. 2011;70:1519]. Ann Rheum Dis. 2010;69:976-986.
38. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs [published correction appears in Ann Rheum Dis. 2011;70:1519]. Ann Rheum Dis. 2010;69:964-975.
39. Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932-1939.
40. Breedveld FC, Emery P, Keystone E, et al. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis. 2004;63:149-155.
41. Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis: a randomized, controlled trial. Ann Intern Med. 1999;130:478-486.
42. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
43. Fleischmann R, Stern R, Iqbal I. Anakinra: an inhibitor of IL-1 for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2004;4:1333-1344.
44. Smolen JS, Kay J, Doyle MK, et al; GO-AFTER Study Investigators. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial [published correction appears in Lancet. 2009;374:1422]. Lancet. 2009;374:210-221.
45. Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane Database Syst Rev. 2009;(1):CD005121.
46. Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition [published correction appears in N Engl J Med. 2005;353:2311]. N Engl J Med. 2005;353:1114-1123.
47. Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435-445.
48. Furst DE, Keystone EC, Braun J, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2010. Ann Rheum Dis. 2011;70(suppl 1):i2-i36.
49. Finckh A, Ciurea A, Brulhart L, et al. Which subgroup of patients with rheumatoid arthritis benefits from switching to rituximab versus alternative anti-tumour necrosis factor (TNF) agents after previous failure of an anti-TNF agent? Ann Rheum Dis. 2010;69:387-393.
50. Sellam J, Hendel-Chavez H, Rouanet S, et al. B cell activation biomarkers as predictive factors for the response to rituximab in rheumatoid arthritis: a six-month, national, multicenter, open-label study. Arthritis Rheum. 2011;63:933-938.
51. Nishimoto N. Interleukin-6 as a therapeutic target in candidate inflammatory diseases. Clin Pharmacol Ther. 2010;87:483-487.
52. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial [published correction appears in Ann Rheum Dis. 2009;68:296]. Ann Rheum Dis. 2008;67:1516-1523.
53. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762-784.
54. Klarenbeek NB, van der Kooij SM, Güler-Yüksel M, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis. 2011;70:315-319.
55. Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2010;62:1515-1526.
56. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR. September 3, 2010. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5934a3.htm. Accessed September 13, 2011.
57. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331.
58. Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials [published correction appears in JAMA. 2006;295:2482]. JAMA. 2006;295:2275-2285.
59. Thompson AE, Rieder SW, Pope JE. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum. 2011;63:1479-1485.
60. Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68:25-32.
61. Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834-4840.
62. Ãstergaard M, Baslund B, Rigby W, et al. Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying antirheumatic drugs: results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum. 2010;62:2227-2238.
63. Investigating clinical efficacy of ofatumumab in adult rheumatoid arthritis (RA) patients who had an inadequate response to MTX therapy. ClinicalTrials.gov. http://www.clinicaltrials.gov/show/NCT00611455. Accessed September 13, 2011.
64. Investigating clinical efficacy of ofatumumab in adult rheumatoid arthritis (RA) patients who had an inadequate response to TNF-α antagonist therapy. ClinicalTrials.gov. http://www.clinicaltrials.gov/show/NCT00603525. Accessed September 13, 2011.
65. Baker KP, Edwards BM, Main SH, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48:3253-3265.
66. A safety and efficacy study of LymphoStat-B⢠(monoclonal anti-BLyS antibody) in subjects with rheumatoid arthritis (RA). ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT00071812. Accessed September 13, 2011.
67. Nestorov I, Munafo A, Papasouliotis O, Visich J. Pharmacokinetics and biological activity of atacicept in patients with rheumatoid arthritis. J Clin Pharmacol. 2008;48:406-417.
68. Tak PP, Thurlings RM, Rossier C, et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. 2008;58:61-72.
69. Study of atacicept in anti-TNF α-naïve patients with moderate to severely active rheumatoid arthritis and an inadequate response to methotrexate. ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT00595413?term=atacicept&rank=3. Accessed September 13, 2011.
70. Kawamura M, McVicar DW, Johnston JA, et al. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci U S A. 1994;91:6374-6378.
71. Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895-1905.
72. Coombs JH, Bloom BJ, Breedveld FC, et al. Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690,550, an orally active Janus kinase (JAK) inhibitor: results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010;69:413-416.
73. Weinblatt ME, Kavanaugh A, Genovese MC, et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N Engl J Med. 2010;363:1303-1312.




