Article
Self-Evidence: When Treatment Based on the Experience of Others is not Enough
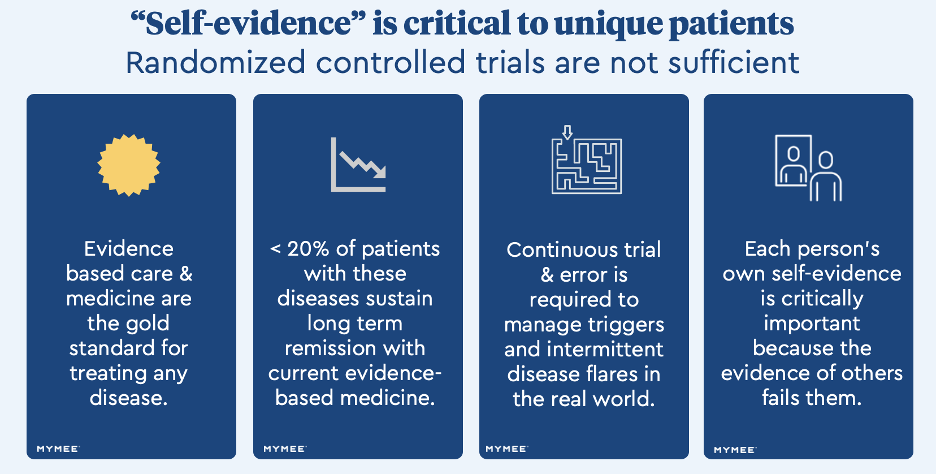
Self-evidence can help rheumatic autoimmune disease patients gain control of symptoms when the standard of care fails them.
Despite the fact that immunosuppressive medications have transformed the care of rheumatic autoimmune patients, there remains a large number who don’t respond to these drugs or are considered partial responders.
Nicole Bundy, MD

Clinical trial data used in Humira’s original regulatory filing to gain FDA approval for psoriatic arthritis is one case in point. In the pivotal study, which was based on results from 151 patients in the treatment arm, 43% continued to experience 81% to over 100% of their disease activity and only 23% were close to or in remission.
How can the standard of care fall short for so many people? Final guidance from The Institute for Clinical and Economic Review (ICER) on JAK inhibitors to treat rheumatoid arthritis attempted to answer this question in 2020, with the headline that clinical data in pivotal Phase III randomized control trials "may not be generalizable to patients in the real world."
Published studies analyzing healthcare claims suggest as many as 85% of rheumatoid arthritis patients taking evidence-based biologics do not sustain remission in year 2. A 2020 ACR survey found that 83% of patients living with rheumatic disease reported activity limitations with 44% facing work limitations. All of this raises the question: Are patients who would have been among the non-responders in the trial taking therapies that are not providing clinical benefit due to a lack of alternatives?
How do we help the millions of patients with diseases like lupus, rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis who are struggling despite evidence-based treatment? Add to this challenge that millions more are now estimated to have Long COVID with rheumatic autoimmune disease biomarkers and unmanageable musculoskeletal symptoms, with no FDA approved treatments.
The published science behind rheumatic autoimmune diseases has made it clear that environmental factors—diet, lifestyle, sleep, stress, pollutants, toxins—can have profound impacts on the severity and frequency of symptoms.
The challenge with these rheumatic autoimmune diseases is that from person to person, there can be vast differences in sensitivities and exposures to different food triggers and other environmental factors, as well as variable requirements for physical activity, stress management and sleep. Such variability helps explain why standard nutritional and activity guidelines that work for one person may, in fact, cause harm to another.
When self-evidence becomes critically important
While most rheumatologists accept that environmental factors play a significant role for their patients, the trick is putting that knowledge into practice, given that every patient is unique.
For patients struggling with poorly controlled symptoms, self-evidence is crucial to understanding what’s driving their disease flares. It requires capturing symptoms, food, activities, habits and other data points to find correlations and create a framework for guided trial and error until patients have enough data to connect the dots between exposures and symptoms, and to understand the impact of cross-reactivity and dosing tolerance between their sensitivities.
Such a deeply personalized approach stands in stark contrast to how evidence-based medicine is practiced today. In her recent op-ed, Karina Davidson, PhD, urges clinicians to broaden the scope of personalized trials to accommodate individual responses to treatment. Self-evidence about personal triggers can similarly inform diet and integrative care interventions to address personal sensitivities as well as optimize treatment by removing external “interference.”
To test the hypothesis that patients could comply with necessary data capture and significantly improve symptom management and severity, Mymee conducted a real-world study among a population that was representative of patients seen by a diverse rheumatology practice. Patients were recruited through the insurance providers Oscar Health and Preferred One, the employer Nielsen, Mount Sinai Hospital’s Post Covid Clinic and peer-to-peer referrals. The group included White, Hispanic, Black and Asian American patients with systemic lupus erythematosus, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, mixed connective tissue disease, rheumatic long COVID, and other autoimmune conditions involving significant rheumatic symptoms. Most (77%) were ages 35-64 years old and 78% were women.
The study evaluated whether patients would capture the self-evidence necessary and adhere to the program. The use of a personalized and adaptive mobile app generated 754 mean observations per patient recording an average 7.4 times/day. This level of engagement continued throughout the program, which averaged 17 weeks from beginning to endpoint and included 14 sessions with a specialized rheumatic autoimmune health and nutrition coach trained on the data analysis platform.
The study demonstrated that personalized interventions based on self-evidence led to clinically meaningful improvements in 10 health-related quality of life domains (as measured by PROMIS tools) including ability to manage symptoms, fatigue, sleep disturbance, pain interference, anxiety, and cognitive function. In the study, improvements were seen across mild, moderate, and severe symptoms as measured by clinically validated test scores, with the most severe symptoms experiencing the greatest improvements.
Putting self-evidence into practice
Leveraging self-evidence to guide personalized interventions can be challenging for patients and clinicians alike.
Rheumatologists are already overwhelmed by demand and short on time- the average appointment lasts between 7-24 minutes. There is not enough time to guide patients through personalized trials to uncover their unique sensitivities and conduct consultations on how to manage those in every real-world scenario that exposes them to potential triggers. But we owe it to patients to teach them about specialized tools and services that can empower them to safely find and navigate hidden triggers without the anxiety that comes with being blindfolded to what may trigger their next flare.
This does not reduce the role of rheumatologists. Patients need drug therapies that can help reduce their autoimmune reactions to environmental factors. When a patient has severe symptoms despite taking the most advanced biologic medications approved by the FDA, it is possible that they are in the subset of patients that do not respond to the drug. If a patient can successfully correlate their symptoms to their trigger exposures, then the rheumatologist can help determine when it makes the most sense to taper or adhere to a drug. Being able to reduce polypharmacy like nonsteroidal anti-inflammatory drugs, steroids, sleep aids, antidepressants, and antianxiety drugs is another huge potential benefit.
Under all circumstances regular check-ups remain critical. Many patients in the Mymee program reported that having data they could share during doctor visits improved their relationship with their rheumatologist. Doctors, patients, and insurance companies all have the same objective: healthier patients, fewer emergency room visits, and ensuring patients can adhere to drugs that offer significant clinical benefit.
As we’ve found, self-evidence gathered through personalized trials offers a new way forward for patients who are vulnerable to hidden triggers that permeate their real world. It can offer patients real hope, empowerment, and a new outlook on their disease, as well as guide meaningful improvements in outcomes.
Nicole Bundy, MD, is a board-certified rheumatologist and the Medical Director at Mymee, a pioneer in self-evidence-based research for people who struggle with symptoms associated with long COVID and rheumatic autoimmune diseases.




